CLIA Kit for Histamine (HA) 
Histamin
- UOM
- FOB US$ 669.00 US$ 955.00 US$ 4,298.00 US$ 8,118.00 US$ 66,850.00
- Quantity
Overview
Properties
- Product No.CCA927Ge
- Organism SpeciesPan-species (General) Same name, Different species.
- ApplicationsChemiluminescent immunoassay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategorySignal transductionInfection immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of Histamine (HA) and the recovery rates were calculated by comparing the measured value to the expected amount of Histamine (HA) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 98-105 | 101 |
| EDTA plasma(n=5) | 83-103 | 90 |
| heparin plasma(n=5) | 86-101 | 89 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Histamine (HA) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Histamine (HA) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Histamine (HA) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 78-96% | 80-97% | 81-89% | 89-104% |
| EDTA plasma(n=5) | 96-103% | 82-90% | 91-105% | 98-105% |
| heparin plasma(n=5) | 78-90% | 90-102% | 81-90% | 88-102% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| Substrate A | 1×10mL | Substrate B | 1×2mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
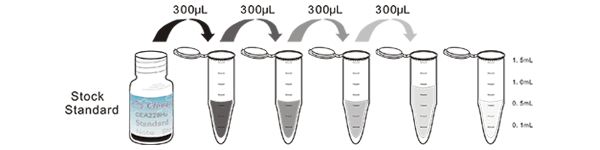
1. Prepare all reagents, samples and standards;
2. Add 50µL standard or sample to each well.
And then add 50µL prepared Detection Reagent A immediately.
Shake and mix. Incubate 1 hour at 37°C;
3. Aspirate and wash 3 times;
4. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
5. Aspirate and wash 5 times;
6. Add 100µL Substrate Solution. Incubate 10 minutes at 37°C;
7. Read RLU value immediately.
Test principle
The microplate provided in this kit has been pre-coated with a monoclonal antibody specific to Histamine (HA). A competitive inhibition reaction is launched between biotin labeled Histamine (HA) and unlabeled Histamine (HA) (Standards or samples) with the pre-coated antibody specific to Histamine (HA). After incubation the unbound conjugate is washed off. Next, avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. The amount of bound HRP conjugate is reverse proportional to the concentration of Histamine (HA) in the sample. Then the mixture of substrate A and B is added to generate glow light emission kinetics. Upon plate development, the intensity of the emitted light is reverse proportional to the Histamine (HA) level in the sample or standard.
Giveaways
Increment services
Citations
- PEST-domain-enriched tyrosine phosphatase and glucocorticoids as regulators of anaphylaxis in micePubmed: 21981059
- Comparison of sensitization between β-lactoglobulin and its hydrolysatesDigital: 875
- Integrative analysis of proteomics and metabolomics of anaphylactoid reaction induced by Xuesaitong injectionPubMed: 26372445
- Expression of inducible nitric oxide synthase in mast cells contributes to the reCavia (Guinea pig )lation of inflammatory cytokines in irritable bowel syndrome with diarrheaPubmed:26940641
- Induction of immune responses and allergic reactions in piglets by injecting glycinindoi:1828051X.2016.1144488
- Effects of Dectin-1 on the mast cells in allergic conjunctivitis and its underlying mechanismfiles:ijcep0019305.pdf
- Proteomics Study on Nonallergic Hypersensitivity Induced by Compound 4880 and Ovalbuminpmc:PMC4734762
- Evaluation of anaphylactoid constituents in vitro and in vivo.pubmed:27984711
- Ultraviolet A photosensitivity profile of dexchlorpheniramine maleate and promethazine-based creams: Anti-inflammatory,antihistaminic, and skin barrier protection propertiesDOI: 10.1111/jocd.12349
- Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat ModelPubmed: 30781605
- Metabolomics analysis of baicalin on ovalbumin-sensitized allergic rhinitis rats
- MOTION SICKNESS INDUCES PHYSIOLOGICAL AND NEURONAL ALTERATIONS IN MOUSE MODEL
- Artemisia annua sublingual immunotherapy for seasonal allergic rhinitis: a randomized controlled trialPubmed: 32030780
- Animal models for analysis of hypersensitivity reactions to Shuanghuanglian injectionPubmed: 32302806
- Protective effect of Asarum sieboldii essential oil on ovalbumin induced allergic rhinitis in ratPubmed: 32395767
- A vaccine-based nanosystem for initiating innate immunity and improving tumor immunotherapyPubmed: 32332752
- CCR3‑shRNA promotes apoptosis and inhibits chemotaxis and degranulation of mouse mast cellsPubmed: 32742345
- Multiplexed fluorescence immunoassay combined with magnetic separation using upconversion nanoparticles as multicolor labels for the simultaneous detection of …Pubmed: 32892931
- Study of some hematological and immunological parameters associated with the infection of intestinal parasites in the holy city of Kerbala, Iraq
- Pharmacokinetics and safety of TCMCB07, a melanocortin-4 antagonist peptide in dogs34014033