Multiplex Assay Kit for Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) 
ANX-A2; ANX2L4; CAL1H; LIP2; LPC2; LPC2D; P36; PAP-IV; Protein I; Chromobindin-8; Calpactin-1 heavy chain; Lipocortin II; Placental anticoagulant protein IV
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 450.00 US$ 468.00 US$ 494.00 US$ 528.00 US$ 563.00 US$ 615.00 US$ 693.00 US$ 866.00
- Quantity
Overview
Properties
- Product No.LMB944Ra
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategorySignal transductionTumor immunityBone metabolism
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
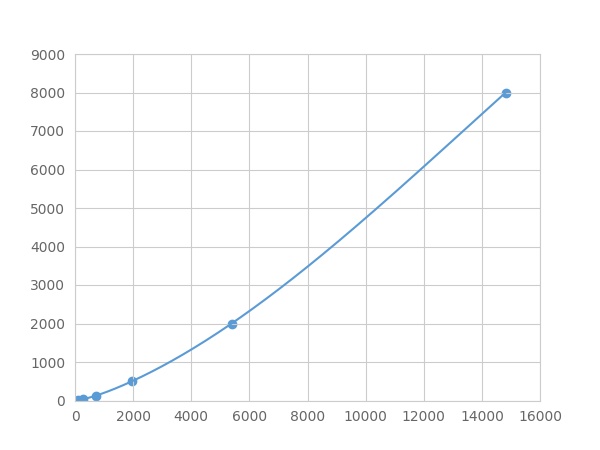
Recovery
Matrices listed below were spiked with certain level of recombinant Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 89-98 | 95 |
| EDTA plasma(n=5) | 96-105 | 99 |
| heparin plasma(n=5) | 89-98 | 93 |
| sodium citrate plasma(n=5) | 95-105 | 101 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Annexin A2 (ANXA2) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 86-101% | 80-104% | 95-103% | 93-101% |
| EDTA plasma(n=5) | 88-98% | 83-98% | 84-98% | 96-105% |
| heparin plasma(n=5) | 78-101% | 91-103% | 87-98% | 79-101% |
| sodium citrate plasma(n=5) | 95-105% | 92-105% | 98-105% | 87-96% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:ANXA2) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
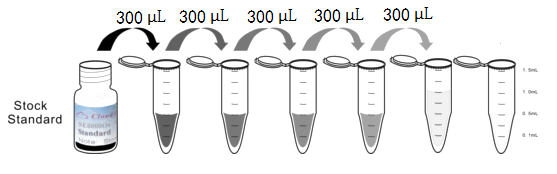
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Subcellular proteome analysis unraveled annexin A2 related to immune liver fibrosisPubMed: 20225235
- Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinomaPubMed: PMC3491596
- Annexin A2 is not a good biomarker for hepatocellular carcinoma in cirrhosisPubMed: PMC3742823
- Annexin A2 Versus Afp as An Efficient Diagnostic Serum Marker for Hepatocellular CarcinomaGhrnet: Source
- Serum annexin A2 levels in acute brucellosis and brucellar spondylodiscitisPubmed:24853056
- Circulating annexin A2 as a biomarker in gastric cancer patients: Correlation with clinical variablesPubmed:25661364
- Serum Levels of Annexin A2 as a Candidate Biomarker for Hepatic Fibrosis in Patients With Chronic Hepatitis BPubMed: 26587036
- Up-regulation of annexin A2 expression predicates advanced clinicopathological features and poor prognosis in hepatocellular carcinomaPubMed: 26109000
- CirculatingPubMed: 25661364
- Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinomaPubMed: 26189841
- Stairs instead of elevators at the workplace decreases PCSK9 levels in a healthy populationPubMed: 26081791
- Serum Annexin A2 Level Is Associated With Diagnosis and Prognosis in Patients With OralSquamous Cell Carcinoma.pubmed:27889534
- Assessment of annexin A5 and annexin A2 levels as biomarkers for pre-eclampsia: A pilot study.pubmed:28501283
- Epigallocatechin gallate induces an up-regulation of LDL receptor accompanied by a reduction of PCSK9 via the annexin A2-independent pathway in HepG2 cells.pubmed:28181408
- Expression of annexin A2 in adenomyosis and dysmenorrheaPubmed: 31183557
- Extracellular Vesicles-Based Biomarkers Represent a Promising Liquid Biopsy in Endometrial CancerPubmed: 31842290
- PLASMA BIOMARKER FOR OVARIAN CANCER
- Diagnostic Value of Plasma Annexin A2 in Early-Stage High-Grade Serous Ovarian Cancer. Diagnostics 2021, 11, 6933406648
- Search for Effective Serum Tumor Markers for Early Diagnosis of Hepatocellular Carcinoma Associated with Hepatitis C
- Bufalin Induced Mitochondrial Dysfunction Promotes Apoptosis of Glioma Cells by Regulating Annexin A2 and DRP1 Proteins
- Bufalin induces mitochondrial dysfunction and promotes apoptosis of glioma cells by regulating Annexin A2 and DRP1 protein expression34376212