Multiplex Assay Kit for Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) 
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 449.00 US$ 467.00 US$ 492.00 US$ 527.00 US$ 562.00 US$ 613.00 US$ 691.00 US$ 864.00
- Quantity
Overview
Properties
- Product No.LMA388Ca
- Organism SpeciesCanis familiaris; Canine (Dog) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryInfection immunityImmune moleculeAutoimmunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 84-92 | 88 |
| EDTA plasma(n=5) | 80-89 | 83 |
| heparin plasma(n=5) | 80-98 | 89 |
| sodium citrate plasma(n=5) | 81-96 | 87 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
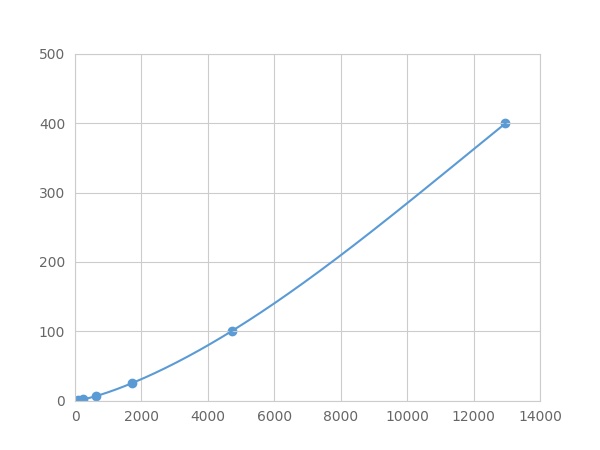
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Complement Component 5a (C5a) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 80-91% | 88-104% | 94-101% | 83-98% |
| EDTA plasma(n=5) | 95-105% | 80-102% | 95-105% | 85-95% |
| heparin plasma(n=5) | 88-95% | 79-91% | 98-105% | 93-103% |
| sodium citrate plasma(n=5) | 99-105% | 81-95% | 86-93% | 89-98% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:C5a) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
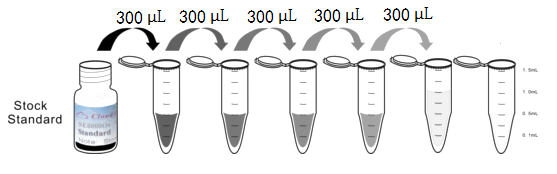
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Cathepsin D is released after severe tissue trauma in vivo and is capable of generating C5a in vitroScienceDirect: S0161589011008297
- Elevated complement factor C5a in maternal and umbilical cord plasma in preeclampsiaPubMed: 23415845
- Complement component 5 contributes to poor disease outcome in humans and mice with pneumococcal meningitisPubMed: PMC3195471
- Efficacy of a synthetic antimicrobial peptidomimetic versus vancomycin in a Staphylococcus epidermidis device-related murine peritonitis modelPubmed: 23645587
- Conversion from external fixator to intramedullary nail causes a second hit and impairs fracture healing in a severe trauma modelPubmed: 23070742
- Therapeutic effects of sesame oil on monosodium urate crystal-induced acute inflammatory response in ratsPubmed: 24353977
- Evaluation of the Markers of Inflammation in the Umbilical Cord Blood of Newborns of Mothers with ThrombophiliaPubmed:25209155
- Complement Split Products C3a/C5a and Receptors: Are They Regulated by Circulating Angiotensin II Type 1 Receptor Autoantibody in Severe Preeclampsia?PubMed: 26485247
- Acute and prolonged complement activation in the central nervous system during herpes simplex encephalitisPubmed:27235358
- Consistency and pathophysiological characterization of a rat polymicrobial sepsis model via the improved cecal ligation and puncture surgeryPubmed:26802602
- Dosagem de frações ativadas do sistema complemento em empiema induzido em ratos10183
- Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsiapubmed:27511439
- Complement C5a/C5aR pathway potentiates the pathogenesis Q5 of gastric cancer by down-regulating p21 expressionpubmed:29031586
- Serum complement factor 5a levels are associated with nonalcoholic fatty liver disease in obese childrenDOI: 10.1111/apa.14106
- Splenectomy modulates early immuno-inflammatory responses to trauma-hemorrhage and protects mice against secondary sepsisPubmed: 30291296
- The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in TriathletesPubmed: 30736479
- Intrathecal complement activation by the classical pathway in tick-borne encephalitisPubmed: 30850976
- Sepsis causes right ventricular myocardial inflammation independent of pulmonary hypertension in a porcine sepsis modelPubmed: 31247004
- C5a/C5aR Pathway is Critical for the Pathogenesis of PsoriasisPubmed: 31447855
- Novel evidence that an alternative complement cascade pathway is involved in optimal mobilization of hematopoietic stem/progenitor cells in Nlrp3 …
- C5aR deficiency attenuates the breast cancer development via the p38/p21 axisPubmed: 32669478
- A Novel Evidence That Mannan Binding Lectin (MBL) Pathway of Complement Cascade Activation is Involved in Homing and Engraftment of Hematopoietic Stem …Pubmed: 32406006
- Modulation of C5a-C5aR interactions against murine mammary cancer cell line
- C‐reactive protein inhibits C3a/C3aR‐dependent podocyte autophagy in favor of diabetic kidney diseasePubmed:35503088