Multiplex Assay Kit for Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) 
CD274; PDCD1LG1; B7-H; B7H1; PD-L1; PDCD1L1; PDCD1-LG1; Programed Death Ligand 1
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 393.00 US$ 408.00 US$ 431.00 US$ 461.00 US$ 491.00 US$ 537.00 US$ 605.00 US$ 756.00
- Quantity
Overview
Properties
- Product No.LMA788Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategorySignal transductionTumor immunityInfection immunityImmune moleculeAutoimmunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 87-101 | 92 |
| EDTA plasma(n=5) | 91-99 | 95 |
| heparin plasma(n=5) | 80-90 | 86 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Programmed Cell Death Protein 1 Ligand 1 (PDL1) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 86-99% | 85-99% | 93-101% | 78-104% |
| EDTA plasma(n=5) | 82-97% | 98-105% | 89-98% | 85-103% |
| heparin plasma(n=5) | 78-95% | 90-99% | 90-101% | 98-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:PDL1) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
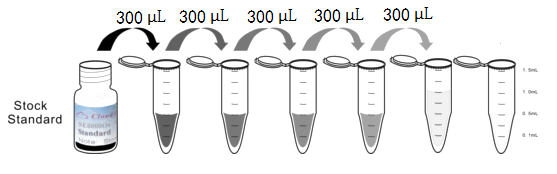
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implicationsNCBI: PMC3937742
- High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial.Pubmed:24732592
- PD‐L1 gene polymorphisms and low serum level of PD‐L1 protein are associated to type 1 diabetes in ChilePubmed:24816853
- Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based studyPubMed: 26686046
- High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis.Pubmed:27039170
- Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancerarticle:10.1007
- PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphomapubmed:27737703
- High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer.pubmed:28212990
- Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predictssurvival in advanced biliary tract cancer patients treated with palliative chemotherapy.pubmed:27780932
- High post-treatment serum levels of soluble programmed cell death ligand 1 predict earlyrelapse and poor prognosis in extranodal NK/T cell lymphoma patients.pubmed:27105512
- Soluble PD-L1: A biomarker to predict progression of autologous transplantation in patients with multiple myelomapubmed:27566569
- Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy.pubmed:28349165
- Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patientsleu2016385a
- Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy.pubmed:28207525
- Inverse correlation of soluble programmed cell death-1 ligand-1 (sPD-L1) with eosinophil count and clinical severity in allergic rhinitis patientspubmed:27617656
- Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapyPMC5319514
- Soluble Programed Death Receptor 1 Ligand (sPD-L1) in Pleural Fluid of Patients with Malignant Pleural Mesothelioma (MPM)Pdf:10.1164
- Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non–Small-cell Lung CancerPubmed:29859759
- Increased levels of soluble programmed death ligand 1 associate with malignancy in patients with dermatomyositisPubmed:29419471
- High Serum Level of Soluble Programmed Death Ligand 1 is Associated With a Poor Prognosis in Hodgkin LymphomaPubmed:29698935
- Increased levels of soluble co-stimulatory molecule PD-L1 (B7-H1) in the plasma of viraemic HIV-1+ individualsPubmed: 30236481
- Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinomaPubmed: 30267213
- Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between …Pubmed: 30565045
- Identification of programmed cell death 1 and its ligand in the testicular tissue of micePubmed: 30578744
- Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated …Pubmed: 30807610
- Elevated levels of soluble PD-L1 are associated with reduced recurrence in papillary thyroid cancerPubmed: 31252406
- Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancerPubmed: 31366979
- The Clinical Significance of Soluble PD-1 and PD-L1 in Lung CancerPubmed: 31675543
- Clinical implications of APOBEC3A and 3B expression in patients with breast cancerPubmed: 32176735
- Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control StudyPubmed: 32085544
- Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphomaPubmed: 32054467
- The prognostic role of soluble transforming growth factor‐β and its correlation with soluble programmed death‐ligand 1 in biliary tract cancerPubmed: 32780918
- Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patientsPubmed: 32577816
- Soluble PD-L1: a potential immune marker for HIV-1 infection and virological failurePubmed: 32443313
- The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte33688997
- Prognostic prospect of soluble programmed cell death ligand-1 (sPD-L1) in cancer management34180502
- Microglial PD‐1 stimulation by astrocytic PD‐L1 suppresses neuroinflammation and Alzheimer's disease pathology34825707
- A Novel Small Cyclic Peptide-Based 68Ga-Radiotracer for Positron Emission Tomography Imaging of PD-L1 Expression in Tumors34910492
- Macrophage Associated Immune Checkpoint CD47 Blocking Ameliorates EndometriosisPubmed:35404426
- Microbial hydrogen “manufactory” for enhanced gas therapy and self-activated immunotherapy via reduced immune escapePubmed:35705974
- Assessment of the RANTES Level Correlation and Selected Inflammatory and Pro-Angiogenic Molecules Evaluation of Their Influence on CRC Clinical Features: A …Pubmed:35208526