Multiplex Assay Kit for Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) 
APCS; PTX2; Pentaxin-Related; 9.5S Alpha-1-Glycoprotein
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 415.00 US$ 431.00 US$ 455.00 US$ 487.00 US$ 519.00 US$ 567.00 US$ 638.00 US$ 798.00
- Quantity
Overview
Properties
- Product No.LMB539Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryInfection immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 97-105 | 101 |
| EDTA plasma(n=5) | 93-101 | 97 |
| heparin plasma(n=5) | 80-95 | 84 |
| sodium citrate plasma(n=5) | 90-102 | 97 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
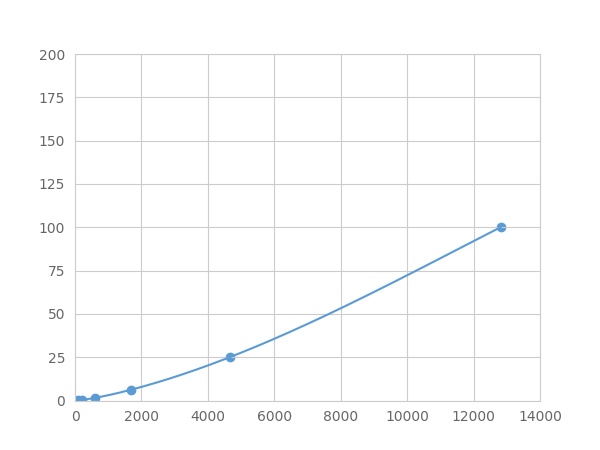
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Serum Amyloid P Component (SAP) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 81-88% | 86-98% | 84-93% | 78-97% |
| EDTA plasma(n=5) | 94-102% | 93-104% | 83-93% | 81-94% |
| heparin plasma(n=5) | 95-104% | 80-92% | 96-103% | 89-97% |
| sodium citrate plasma(n=5) | 83-105% | 99-105% | 93-101% | 86-101% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:SAP) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Circulation levels of acute phase proteins in patients with Takayasu arteritisPubMed: 20100644
- Combined Proteomic and Metabolomic Profiling of Serum Reveals Association of the Complement System with Obesity and Identifies Novel Markers of Body Fat Mass ChangesACS: pr2005555
- Identification of candidate biomarkers for hepatocellular carcinoma in plasma of HCV-infected cirrhotic patients by 3-D DIGEPubmed: 23944848
- Targeted proteomics predict a sustained complete-response after transarterial chemoembolization and clinical outcomes in patients with hepatocellular carcinoma: a prospective cohort studypubmed:28112944
- Amniotic fluid pentraxins: Potential early markers for identifying intra‐amniotic inflammatory complications in preterm pre‐labor rupture of membranespubmed:29193454
- Serum amyloid P component therapeutically attenuates atherosclerosis in mice via its effects on macrophagesPubmed:29930724
- Proteopathic lesions in the brain of a super©\aged chimpanzee (Pan troglodytes)34096618
- Clinical significance of differential serum-signatures for early prediction of severe dengue among Eastern Indian patientsPubmed:35348620