Active Heparanase (HPSE) 
HPA; HPR1; HPSE1; HSE1; Endo-glucoronidase;
- UOM
- FOB US$ 302.00 US$ 754.00 US$ 1,508.00 US$ 4,524.00 US$ 11,310.00
- Quantity
Overview
Properties
- Product No.APA711Hu61
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsCell culture; Activity Assays.
Research use only - DownloadInstruction Manual
- CategoryEnzyme & Kinase
- Buffer FormulationPBS, pH7.4, containing 5% Trehalose.
- Traits Freeze-dried powder, Purity > 95%
- Isoelectric Point9.5
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Activity test

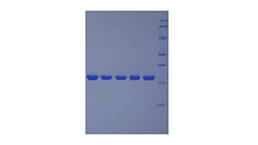
Heparanase (HPSE) selectively cleaves heparan sulfate at specific sites on heparan sulfate proteoglycans (HSPGs). HPSE facilitates cell migration associated with metastasis, wound healing and inflammation. An increase in its activity is associated with an increase in VEGF activity, which further enhances angiogenesis. HPSE also enhances shedding of syndecans and increases endothelial invasion and angiogenesis in myelomas. It acts as a procoagulant by increasing the generation of activation factor X in the presence of tissue factor and activation factor VII. In addition, it increases cell adhesion to the extracellular matrix (ECM), independent of its enzymatic activity. HPSE is highly expressed in placenta and spleen and weakly expressed in lymph node, thymus, peripheral blood leukocytes, bone marrow, endothelial cells, fetal liver and tumor tissues. The enzyme activity of recombinant human HPSE was assayed in an ELISA format using non-reducing end biotinylated heparan sulfate on recombinant human syndecan 4 as a substrate. Briefly, combining 10 µL of different concentrations of rhHPSE with 10 µL of the biotinylated-syndecan 4 in a vial (10 µL of assay buffer and 10 µL of biotinylated-syndecan 4 as a negative control) and incubated for 2h at 37℃. After incubation, 220 µL of PBS, with 1% BSA (pH7.4) was added to each reaction, then loading 100 ul of each sample onto the anti-syndecan 4 pAb-coated microtiter wells and incubated for 1h at 37℃. Wells were washed with PBST 3 times and incubation with Streptavidin-HRP for 30min, then wells were aspirated and washed 5 times. With the addition of substrate solution, wells were incubated 15-25 minutes at 37℃. Finally, add 50 µl stop solution to the wells and read at 450nm immediately. As a result, 62.5 ng of recombinant human HPSE digestion will result in >50% of OD reduction compared with the negative control.
Usage
Reconstitute in ddH2O to a concentration less than or equal to 0.1mg/mL. Do not vortex.
Storage
Avoid repeated freeze/thaw cycles. Store at 2-8°C for one month. Aliquot and store at -80°C for 12 months.
Stability
The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37°C for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition.
Increment services
-
 BCA Protein Quantification Kit
BCA Protein Quantification Kit
-
 Molecular Mass Marker for Protein
Molecular Mass Marker for Protein
-
 Monoclonal Antibody Customized Service
Monoclonal Antibody Customized Service
-
 Polyclonal Antibody Customized Service
Polyclonal Antibody Customized Service
-
 Protein Activity Test Experiment Service
Protein Activity Test Experiment Service
-
 Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
-
 Buffer
Buffer
-
 Lentivirus Packaging Experiment Service
Lentivirus Packaging Experiment Service
-
 Adenovirus Packaging Experiment Service
Adenovirus Packaging Experiment Service
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Spike RBD Protein (S-RBD)
Spike RBD Protein (S-RBD)
-
 Protein G
Protein G
-
 Protein A
Protein A
Citations
- PG545, a Heparan Sulfate Mimetic, Reduces Heparanase Expression In Vivo, Blocks Spontaneous Metastases and Enhances Overall Survival in the 4T1 Breast Carcinoma ModelPlosone: Source
- Assessment of heparanase and heparin-binding growth and angiogenesis factors in the uterine cavity ? uid in women with impaired reproductionNowinylekarskie:Source
- Unfractionated heparin attenuates intestinal injury in mouse model of sepsis by inhibiting heparanasePubMed: 26191183
- PI-88 inhibits postoperative recurrence of hepatocellular carcinoma via disrupting the surge of heparanase after liver resectionPubMed: 26415733
- Assessment of heparanase and heparin-binding growth and angiogenesis factors in the uterine cavity fluid in women with impaired reproductionarticle:84
- Plasma heparanase is associated with blood glucose levels but not urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage pubmed:28994624