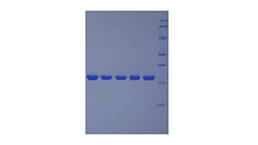
Recombinant Fibronectin Type III Domain Containing Protein 5 (FNDC5) 
FRCP2; Irisin; Fibronectin type III repeat-containing protein 2
Overview
Properties
- Product No.RPN576Hu01
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
-
Applications
Positive Control; Immunogen; SDS-PAGE; WB.
If bio-activity of the protein is needed, please check active protein.
Research use only - Downloadn/a
- CategoryCD & Adhesion molecule
- Source Prokaryotic expression, Host E.coli
- Endotoxin Level<1.0EU per 1µg (determined by the LAL method)
- Subcellular LocationMembrane
- Molecular Mass n/a, Accurate n/a(Analysis of differences refer to the manual)
- Residues & Tags
- Buffer FormulationPBS, pH7.4, containing 0.01% SKL, 5% Trehalose.
- Traits Freeze-dried powder, Purity > 90%
- Isoelectric Pointn/a
Share your citation
Upload your experimental result
Review
Leave a message
Loading...
Sign into your account
Share a new citation as an author
Upload your experimental result
Review
Please attach serial No. on instruction manual


Contact us
Please fill in the blank.
Name*
Organization
Address
E-mail address*
Telephone
Inquiry*
Verification code*
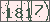
Sequence

Usage
Reconstitute in PBS or others.
Storage
Avoid repeated freeze/thaw cycles. Store at 2-8°C for one month. Aliquot and store at -80°C for 12 months.
Stability
The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37°C for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition.
Increment services
-
 Endotoxin Removal Kit
Endotoxin Removal Kit
-
 BCA Protein Quantification Kit
BCA Protein Quantification Kit
-
 Protein Labeling Customized Service
Protein Labeling Customized Service
-
 Molecular Mass Marker for Protein
Molecular Mass Marker for Protein
-
 Recombinant Protein Customized Service
Recombinant Protein Customized Service
-
 Monoclonal Antibody Customized Service
Monoclonal Antibody Customized Service
-
 Polyclonal Antibody Customized Service
Polyclonal Antibody Customized Service
-
 Protein Activity Test Experiment Service
Protein Activity Test Experiment Service
-
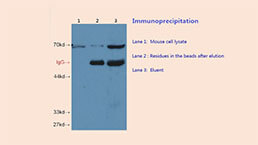 Immunoprecipitation (IP) Experiment Service
Immunoprecipitation (IP) Experiment Service
-
 Buffer
Buffer
-
 Endotoxin Removal Kit II
Endotoxin Removal Kit II
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Spike RBD Protein (S-RBD)
Spike RBD Protein (S-RBD)
-
 Protein G
Protein G
-
 Protein A
Protein A
Citations
- Serum irisin levels in new-onset type 2 diabetesPubMed: 23369227
- Lower circulating irisin is associated with type 2 diabetes mellitusPubmed: 23619195
- Inconsistency in circulating irisin levels: what is really happeningPubmed:24459033
- Leptin administration activates irisin-induced myogenesis via nitric oxide-dependent mechanisms, but reduces its effect on subcutaneous fat browning in micePubmed:25199621
- Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese micePubmed:25576856
- Lower irisin level in patients with type 2 diabetes mellitus: A case-control study and meta-analysisPubMed: 25494632
- Maternal circulating levels of irisin in intrahepatic cholestasis of pregnancyPubMed: 26689349
- Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-AnalysisPubmed:27300472
- Physiology and role of irisin in glucose homeostasis.pubmed:28211512
- Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese micepubmed:27638193
- Irisin stimulates cell proliferation and invasion by targeting the PI3K/AKT pathway in human hepatocellular carcinoma pubmed:28867187
- Differential actions of PPAR-α and PPAR-β/δ on beige adipocyte formation: A study in the subcutaneous white adipose tissue of obese male micePubmed:29351550
- The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer.Pubmed:29522296
- Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative …Doi: 10.1016/j.redox.2018.10.019
- Low serum irisin concentration is associated with poor outcomes in patients with acute pancreatitis and irisin administration protects against experimental acute …Pubmed: 31250660
- Maternal serum, placental, and umbilical venous blood irisin levels in intrahepatic cholestasis of pregnancyPubmed: 31590596
- Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitisPubmed: 32199226
- Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver InjuryPubmed: 31976032
- Serum Irisin Predicts Posthepatectomy Complications in Patients with Hepatocellular CarcinomaPubmed: 31976024
- The myokine Irisin: localization and effects in swine late medium and large antral ovarian folliclePubmed: 33120167
- The Combination of Aerobic and Resistance Exercise Induces Weight Loss via the PGC-1α/Irisin/UCP-1 Pathway
- Evaluation of a recombinant bacillus calmette-guérin vaccine expressing P39-L7/L12 of Brucella melitensis: An immunization strategy against brucellosis in BALB/c …
- Salivary Irisin: potential inflammatory biomarker in recurrent apthous stomatitis patients33755963
- Pattern of Adiponectin, Osteocalcin, Irisin, FGF-21, and MCP-1 According to the Body Size Phenotype: Could They Be Markers of Metabolic Health in Mexican-Mestizo …34822430
- Time-restricted feeding prevents metabolic diseases through the regulation of galanin/GALR1 expression in the hypothalamus of mice34370270
- Eight-week exercise program improved the quality of life of Alzheimer's patients through functional, cognitive, and biochemical parametersPubmed:35604540
- Irisin attenuates sepsis-induced cardiac dysfunction by attenuating inflammation-induced pyroptosis through a mitochondrial ubiquitin ligase-dependent …Pubmed:35653888
- Interaction of nitric oxide synthase and mitochondrial ATP-sensitive potassium channels in protective impacts of combination therapy with irisin …