ELISA Kit for Anterior Gradient 2 (AGR2) 
GOB4; HAG2; XAG2; AG2; PDIA17; HPC8; Anterior Gradient Protein 2; Protein Disulfide Isomerase Family A,Member 17; Secreted cement gland protein XAG-2 homolog
- UOM
- FOB US$ 490.00 US$ 700.00 US$ 3,150.00 US$ 5,950.00 US$ 49,000.00
- Quantity
Overview
Properties
- Product No.SEC285Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsEnzyme-linked immunosorbent assay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryTumor immunityReproductive scienceBone metabolism
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Anterior Gradient 2 (AGR2) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Anterior Gradient 2 (AGR2) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
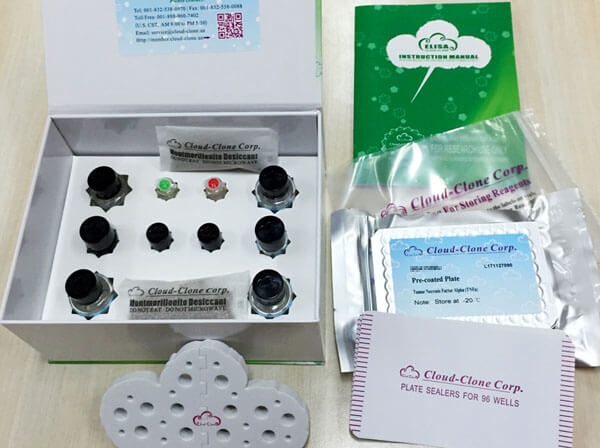
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
Test principle
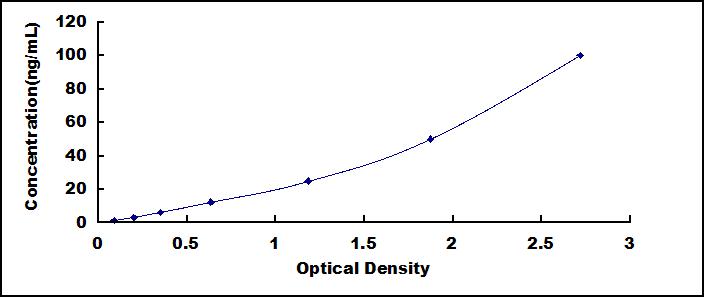
The test principle applied in this kit is Sandwich enzyme immunoassay. The microtiter plate provided in this kit has been pre-coated with an antibody specific to Anterior Gradient 2 (AGR2). Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated antibody specific to Anterior Gradient 2 (AGR2). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain Anterior Gradient 2 (AGR2), biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of Anterior Gradient 2 (AGR2) in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Giveaways
Increment services
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
Citations
- Serum AGR2 as an early diagnostic and postoperative prognostic biomarker of human lung adenocarcinomaMetaPress: n7137r6271u54118
- Integrated Proteomic Profiling of Cell Line Conditioned Media and Pancreatic Juice for the Identification of Pancreatic Cancer BiomarkersPubMed: PMC3205865
- Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotypePubmed: 22911164
- Validation of four candidate pancreatic cancer serological biomarkers that improve the performance of CA19.9Pubmed: 24007603
- Validation of Biomarkers That Complement CA19.9 in Detecting Early Pancreatic CancerPubmed:25239611
- AGR3 in Breast Cancer: Prognostic Impact and Suitable Serum-Based Biomarker for Early Cancer DetectionPubMed: 25875093
- Serum AGR2 as a useful biomarker for pituitary adenomasS0303846717300045
- Identification, characterization and application of a new peptide against anterior gradient homolog 2 (AGR2)Pubmed:29937991
- Anterior gradient protein 2 is a marker of tumor aggressiveness in breast cancer and favors chemotherapy‑induced senescence escape34913074
- Proteome profiling of enzalutamide‐resistant cell lines and serum analysis identified ALCAM as marker of resistance in castration‐resistant prostate cancerPubmed:35689436