Canine Model for Myocardial Infarction (MI) 
AMI; Acute myocardial infarction; Heart attack
- UOM
- FOB US$
- Quantity
Overview
Properties
- Product No.DSI504Ca01
- Organism SpeciesCanis familiaris; Canine (Dog) Same name, Different species.
- Applicationsn/a
Research use only - Downloadn/a
- CategoryCirculatory system
- Prototype SpeciesHuman
- SourceInduced by electrical stimulation of the coronary artery
- Model Animal StrainsClosed group, general level, age 6 to 9 months old, weight: 6-10kg.
- Modeling GroupingRandomly divided into six group: Control group, Model group, Positive drug group and Test drug group(low,medium,high).
- Modeling Periodn/a
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Modeling Method
Intravenous anesthesia with 3% pentobarbital sodium at a dose of 30mg/kg, then insert the tracheal tube. Fix the dogs to right lying position, isolate femoral artery and femoral vein to seperately used to measure peripheral arterial pressure and to the test sample and supplemental anesthesia [3% pentobarbital sodium 10ml by adding 500 ml of saline) at a rate of (5 ml/kg·h). Isolated right jugular vein for intubation of heparin. From the 4th, 5th intercostal thoracotomy, cut the pericardium, expose the heart, separate the left branch (LCX). From the root to the first main branch, ligature all the rest of the small branches, separation length is nearly 2cm, hook the appropriate electromagnetic flowmeter probe (2mm), continuously record the left-handed blood flow. Draw a filament line under LCX, lift the thread to block the blood flow for 20s, observe and record the traffic changes. Lay down the thread so that the blood is fully filled and the flow is the peak flow. Place 2-3 small silver clip on the LCX, adjust the silver folder, so that blood flow reduced to about 30% of peak flow(reduce to 8ml/min in this test). Start the electrical stimulation when the flow is stable. The electric stimulation needle should be made of stainless steel, the tip of the electrode penetrates the LCX vessel wall and extends into the lumen and fixed to determine that its tip is in intimate contact with the arterial intima. Electronically stimulated DC current of 150 μA, continued until LCX blood flow reduced to 0 and continued for 3mins. Electrical stimulation time is at least 15mins, and record the time for the blood flow to block the time. In the screening of samples, after treatment to see the recovery time for the start of administration to LCX flow recovery to the electrical stimulation before the flow of 1/3 of the time. The resistance time is from re-pass to LCX traffic reduce to zero again. At the end of the trial, the animals were sacrificed and cut the LCX segment long enough to open, remove the thrombus for weighing.
Model evaluation
Pathological results
Cytokines level
Statistical analysis
SPSS software is used for statistical analysis, measurement data to mean ± standard deviation (x ±s), using t test and single factor analysis of variance for group comparison , P<0.05 indicates there was a significant difference, P<0.01 indicates there are very significant differences.
Giveaways
Increment services
-
 Tissue/Sections Customized Service
Tissue/Sections Customized Service
-
 Serums Customized Service
Serums Customized Service
-
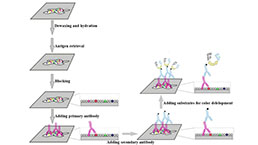 Immunohistochemistry (IHC) Experiment Service
Immunohistochemistry (IHC) Experiment Service
-
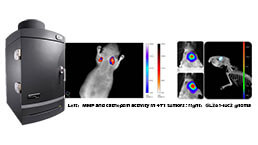 Small Animal In Vivo Imaging Experiment Service
Small Animal In Vivo Imaging Experiment Service
-
 Small Animal Micro CT Imaging Experiment Service
Small Animal Micro CT Imaging Experiment Service
-
 Small Animal MRI Imaging Experiment Service
Small Animal MRI Imaging Experiment Service
-
 Small Animal Ultrasound Imaging Experiment Service
Small Animal Ultrasound Imaging Experiment Service
-
 Transmission Electron Microscopy (TEM) Experiment Service
Transmission Electron Microscopy (TEM) Experiment Service
-
 Scanning Electron Microscope (SEM) Experiment Service
Scanning Electron Microscope (SEM) Experiment Service
-
 Learning and Memory Behavioral Experiment Service
Learning and Memory Behavioral Experiment Service
-
 Anxiety and Depression Behavioral Experiment Service
Anxiety and Depression Behavioral Experiment Service
-
 Drug Addiction Behavioral Experiment Service
Drug Addiction Behavioral Experiment Service
-
 Pain Behavioral Experiment Service
Pain Behavioral Experiment Service
-
 Neuropsychiatric Disorder Behavioral Experiment Service
Neuropsychiatric Disorder Behavioral Experiment Service
-
 Fatigue Behavioral Experiment Service
Fatigue Behavioral Experiment Service
-
 Nitric Oxide Assay Kit (A012)
Nitric Oxide Assay Kit (A012)
-
 Nitric Oxide Assay Kit (A013-2)
Nitric Oxide Assay Kit (A013-2)
-
 Total Anti-Oxidative Capability Assay Kit(A015-2)
Total Anti-Oxidative Capability Assay Kit(A015-2)
-
 Total Anti-Oxidative Capability Assay Kit (A015-1)
Total Anti-Oxidative Capability Assay Kit (A015-1)
-
 Superoxide Dismutase Assay Kit
Superoxide Dismutase Assay Kit
-
 Fructose Assay Kit (A085)
Fructose Assay Kit (A085)
-
 Citric Acid Assay Kit (A128 )
Citric Acid Assay Kit (A128 )
-
 Catalase Assay Kit
Catalase Assay Kit
-
 Malondialdehyde Assay Kit
Malondialdehyde Assay Kit
-
 Glutathione S-Transferase Assay Kit
Glutathione S-Transferase Assay Kit
-
 Microscale Reduced Glutathione assay kit
Microscale Reduced Glutathione assay kit
-
 Glutathione Reductase Activity Coefficient Assay Kit
Glutathione Reductase Activity Coefficient Assay Kit
-
 Angiotensin Converting Enzyme Kit
Angiotensin Converting Enzyme Kit
-
 Glutathione Peroxidase (GSH-PX) Assay Kit
Glutathione Peroxidase (GSH-PX) Assay Kit
-
 Cloud-Clone Multiplex assay kits
Cloud-Clone Multiplex assay kits