Rat Model for Chronic Obstructive Pulmonary Disease (COPD) 
- UOM
- FOB US$ 240.00
- Quantity
Overview
Properties
- Product No.DSI557Ra02
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- ApplicationsDiesase model
Research use only - Downloadn/a
- Category
- Prototype SpeciesHuman
- SourceInduced by smoking
- Model Animal StrainsSD Rats(SPF), healthy, male, body weight 190g~230g.
- Modeling GroupingRandomly divided into six group: Control group, Model group, Positive drug group and Test drug group.
- Modeling Period4 weeks
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Modeling Method
1. Narcotize rats with ether at day 1 and day 14, expose its larynx, replace the tracheal tube with a venous cannula, inject 200μg/kg lipopolysaccharide (LPS) into rats trachea, then revolve rats vertically 10-20s to diffuse LPS at lung evenly.
2.At days 2-13 and days 15-28, fresh smoke was steadily provided to the closed glass box (70cm×60cm×60cm) by 15 cigarettes per day, to maintain smokescope about 100-120 mg/m3. After smoking, stimulate immediately with 5℃ cool air for 1 hour. End of modeling at day 28.
Model evaluation
1.Observe activity, hair, appetite, breathe, weight of rats in each group. Weigh at 1 hour before feeding every week to observe the change of weight.
2.RT-qPCR Analysis
Collect rats PBMC and lung tissue, extract RNA, detect the level of Foxp3、RORγT by RT-PCR.
3.Western blot Analysis
Collect rats PBMC and lung tissue, extract protein, detect the level of TAZ by Western blot.
4. Flow cytometry for Peripheral blood T lymphocytes
Collect 2ml rat abdominal aortic blood in each group, extract peripheral blood mononuclear cells (PBMC) cell suspensions from rat peripheral blood lymphocytes.
(1)Detection of Th17: Add phorbol ester (50ng/ml), ionomycin (1μg/ml) and coban (2μmol/L) into the cell suspension. Incubate at 37℃, 5%CO2 for 6 hours, resuspend with 100μL PBS after washing 3 times. Add 10μL CD4 monoclonal antibody, mix it up, incubate at 4℃ without light for 30min. Wash, fix and break. Add 10μL IL-17 monoclonal antibody, incubate at 4℃ without light for 20min. Resuspend with 500μL PBS after washing repeatedly. Proportion of CD4+IL-17+ cells in CD4+T cells were detected by flow cytometry.
Detection of Treg cells: Add 10μl CD4 and CD25 monoclonal antibody into the cell suspension. After mixing, incubate at 4℃ without light for 30min. Wash, fix and break. Add 10μL FoxP3 monoclonal antibody, incubate at 4℃ without light for 20min. Resuspend with 500μL PBS after washed repeatedly. Proportion of CD4+CD25+FoxP3+ cells in CD4+T cells were detected by flow cytometry.
Pathological results
HE staining: The right lung lobe tissue was fixed on the embedded section, and the pathological changes of lung tissue were observed after staining.
Cytokines level
ELISA Analysis
Detect the concentration of IL-17 and IL-10 in serum and bronchoalveolar lavage fluid (BALF).
BALF: After blood collection, open its chest, separate mediastina, inject 3ml saline from trachea to the lung. Every time lavage thrice, collect the douche. Centrifuged it at 4℃, 3000r/min for 15 min, take the supernatant.
Serum: Place whole blood samples collected in the serum separation tube at room temperature for 2 hours or 4℃ overnight. Centrifuge it at 1,000×g for 20 min, take the supernatant. Store the supernatant at -20℃ or -80℃, avoid repeated freezing and thawing.
Statistical analysis
SPSS software is used for statistical analysis, measurement data to mean ± standard deviation (x ±s), using t test and single factor analysis of variance for group comparison , P<0.05 indicates there was a significant difference, P<0.01 indicates there are very significant differences.
Giveaways
Increment services
-
 Tissue/Sections Customized Service
Tissue/Sections Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 Immunohistochemistry (IHC) Experiment Service
Immunohistochemistry (IHC) Experiment Service
-
 Small Animal In Vivo Imaging Experiment Service
Small Animal In Vivo Imaging Experiment Service
-
 Small Animal Micro CT Imaging Experiment Service
Small Animal Micro CT Imaging Experiment Service
-
 Small Animal MRI Imaging Experiment Service
Small Animal MRI Imaging Experiment Service
-
 Small Animal Ultrasound Imaging Experiment Service
Small Animal Ultrasound Imaging Experiment Service
-
 Transmission Electron Microscopy (TEM) Experiment Service
Transmission Electron Microscopy (TEM) Experiment Service
-
 Scanning Electron Microscope (SEM) Experiment Service
Scanning Electron Microscope (SEM) Experiment Service
-
 Learning and Memory Behavioral Experiment Service
Learning and Memory Behavioral Experiment Service
-
 Anxiety and Depression Behavioral Experiment Service
Anxiety and Depression Behavioral Experiment Service
-
 Drug Addiction Behavioral Experiment Service
Drug Addiction Behavioral Experiment Service
-
 Pain Behavioral Experiment Service
Pain Behavioral Experiment Service
-
 Neuropsychiatric Disorder Behavioral Experiment Service
Neuropsychiatric Disorder Behavioral Experiment Service
-
 Fatigue Behavioral Experiment Service
Fatigue Behavioral Experiment Service
-
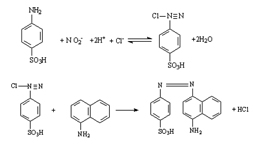 Nitric Oxide Assay Kit (A012)
Nitric Oxide Assay Kit (A012)
-
 Nitric Oxide Assay Kit (A013-2)
Nitric Oxide Assay Kit (A013-2)
-
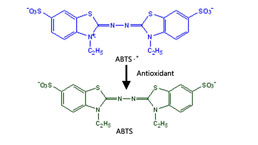 Total Anti-Oxidative Capability Assay Kit(A015-2)
Total Anti-Oxidative Capability Assay Kit(A015-2)
-
 Total Anti-Oxidative Capability Assay Kit (A015-1)
Total Anti-Oxidative Capability Assay Kit (A015-1)
-
 Superoxide Dismutase Assay Kit
Superoxide Dismutase Assay Kit
-
 Fructose Assay Kit (A085)
Fructose Assay Kit (A085)
-
 Citric Acid Assay Kit (A128 )
Citric Acid Assay Kit (A128 )
-
 Catalase Assay Kit
Catalase Assay Kit
-
 Malondialdehyde Assay Kit
Malondialdehyde Assay Kit
-
 Glutathione S-Transferase Assay Kit
Glutathione S-Transferase Assay Kit
-
 Microscale Reduced Glutathione assay kit
Microscale Reduced Glutathione assay kit
-
 Glutathione Reductase Activity Coefficient Assay Kit
Glutathione Reductase Activity Coefficient Assay Kit
-
 Angiotensin Converting Enzyme Kit
Angiotensin Converting Enzyme Kit
-
 Glutathione Peroxidase (GSH-PX) Assay Kit
Glutathione Peroxidase (GSH-PX) Assay Kit
-
 Cloud-Clone Multiplex assay kits
Cloud-Clone Multiplex assay kits
Citations
- Overexpression of histone deacetylase SIRT1 exerts an anti-angiogenic role in diabetic retinopathy via miR-20a elevation and YAP/HIF1α/VEGFA depletionPubmed: 32776826
- Circular RNA circBbs9 promotes PM2. 5-induced lung inflammation in mice via NLRP3 inflammasome activationPubmed: 32707273