CLIA Kit for Amphiregulin (AREG) 
AR; CRDGF; SDGF; Colorectum Cell-Derived Growth Factor; Schwannoma-Derived Growth Factor
- UOM
- FOB US$ 588.00 US$ 840.00 US$ 3,780.00 US$ 7,140.00 US$ 58,800.00
- Quantity
Overview
Properties
- Product No.SCA006Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsChemiluminescent immunoassay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryCytokineTumor immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Amphiregulin (AREG) and the recovery rates were calculated by comparing the measured value to the expected amount of Amphiregulin (AREG) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 90-98 | 94 |
| EDTA plasma(n=5) | 79-99 | 88 |
| heparin plasma(n=5) | 98-105 | 102 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Amphiregulin (AREG) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Amphiregulin (AREG) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Amphiregulin (AREG) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 90-101% | 93-104% | 93-101% | 95-103% |
| EDTA plasma(n=5) | 79-90% | 84-103% | 80-91% | 98-105% |
| heparin plasma(n=5) | 89-97% | 85-99% | 94-105% | 97-104% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| Substrate A | 1×10mL | Substrate B | 1×2mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
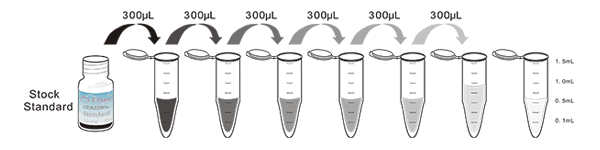
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 100µL Substrate Solution. Incubate 10 minutes at 37°C;
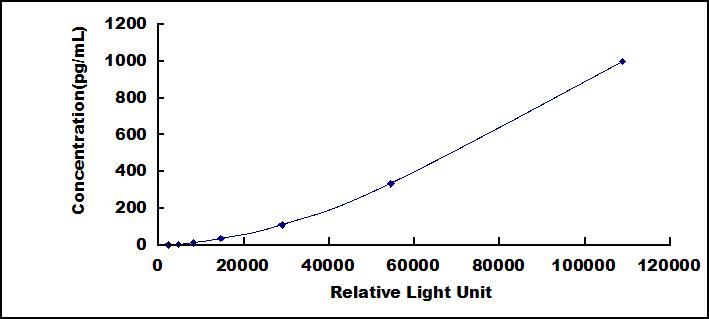
8. Read RLU value immediately.
Test principle
The microplate provided in this kit has been pre-coated with an antibody specific to Amphiregulin (AREG). Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated antibody specific to Amphiregulin (AREG). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then the mixture of substrate A and B is added to generate glow light emission kinetics. Upon plate development, the intensity of the emitted light is proportional to the Amphiregulin (AREG) level in the sample or standard.;
Giveaways
Increment services
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 CLIA Kit Customized Service
CLIA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
Citations
- Amphiregulin and Epiregulin Expression in Colorectal Carcinoma and the Correlation with Clinicopathological CharacteristicsKarger: 315380
- Serum levels of hepatocyte growth factor and epiregulin are associated with the prognosis on anti-EGFR antibody treatment in KRAS wild-type metastatic colorectal cancer.Pubmed:24800946
- Association between serum ligands and the skin toxicity of anti-epidermal growth factor receptor antibody in metastatic colorectal cancerPubMed: 25707609
- Amphiregulin confers trastuzumab resistance via AKT and ERK activation in HER2-positive breast cancerPubMed: 26195282
- Serum level of hepatocyte growth factor is a novel marker of predicting theoutcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancerPubmed:26716644
- Anti-asthmatic activity of alkaloid compounds from Pericarpium Citri Reticulatae (Citrus reticulata 'Chachi')Pubmed: 19363516
- CD9 regulates keratinocyte migration by negatively modulating the sheddase activity of ADAM17
- Patient-derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicinePubmed: 33212364
- Polarization of ADAM17‐driven EGFR signalling in electric field‐guided collective migration of epidermal sheetsPubmed: 33164313