CLIA Kit for Coagulation Factor II (F2) 
FII; TM; PT; Thrombin; Prothrombin; Pro-Thrombin; Activation peptide fragment 1; Activation peptide fragment 2
- UOM
- FOB US$ 588.00 US$ 840.00 US$ 3,780.00 US$ 7,140.00 US$ 58,800.00
- Quantity
Overview
Properties
- Product No.SCA820Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsChemiluminescent immunoassay for Antigen Detection.
Research use only - Downloadn/a
- CategorySignal transductionHematology
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Coagulation Factor II (F2) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Coagulation Factor II (F2) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
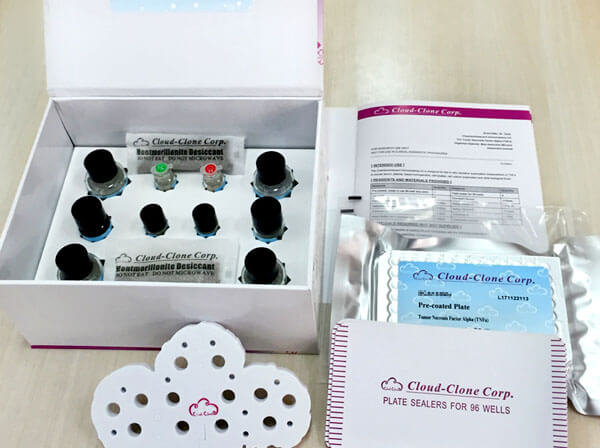
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| Substrate A | 1×10mL | Substrate B | 1×2mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
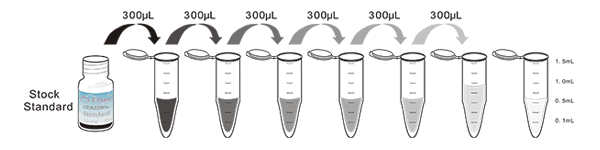
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 100µL Substrate Solution. Incubate 10 minutes at 37°C;
8. Read RLU value immediately.
Test principle
The microplate provided in this kit has been pre-coated with an antibody specific to Coagulation Factor II (F2). Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated antibody specific to Coagulation Factor II (F2). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then the mixture of substrate A and B is added to generate glow light emission kinetics. Upon plate development, the intensity of the emitted light is proportional to the Coagulation Factor II (F2) level in the sample or standard.;
Giveaways
Increment services
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 CLIA Kit Customized Service
CLIA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
Citations
- Upregulation of cytokine signalling in platelets increases risk of thrombophilia in severe COVID-19 patientsPubmed:35180460