CLIA Kit for Immunoglobulin E (IgE) 
IGHE; Immunoglobulin Heavy Constant Epsilon; Ig epsilon chain C region
- UOM
- FOB US$ 635.00 US$ 907.00 US$ 4,082.00 US$ 7,710.00 US$ 63,490.00
- Quantity
Overview
Properties
- Product No.SCA545Po
- Organism SpeciesSus scrofa; Porcine (Pig) Same name, Different species.
- ApplicationsChemiluminescent immunoassay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryInfection immunityImmune moleculeHematologyAutoimmunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Immunoglobulin E (IgE) and the recovery rates were calculated by comparing the measured value to the expected amount of Immunoglobulin E (IgE) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 81-90 | 87 |
| EDTA plasma(n=5) | 80-92 | 89 |
| heparin plasma(n=5) | 81-94 | 87 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Immunoglobulin E (IgE) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Immunoglobulin E (IgE) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Immunoglobulin E (IgE) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 95-102% | 85-92% | 86-98% | 90-98% |
| EDTA plasma(n=5) | 93-105% | 78-96% | 94-105% | 91-98% |
| heparin plasma(n=5) | 92-101% | 80-101% | 79-97% | 80-90% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| Substrate A | 1×10mL | Substrate B | 1×2mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 100µL Substrate Solution. Incubate 10 minutes at 37°C;
8. Read RLU value immediately.
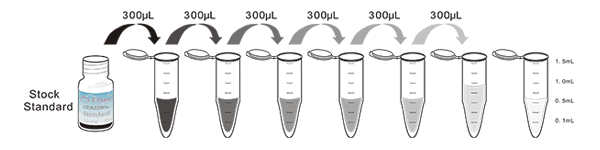
Test principle
The microplate provided in this kit has been pre-coated with an antibody specific to Immunoglobulin E (IgE). Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated antibody specific to Immunoglobulin E (IgE). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then the mixture of substrate A and B is added to generate glow light emission kinetics. Upon plate development, the intensity of the emitted light is proportional to the Immunoglobulin E (IgE) level in the sample or standard.;
Giveaways
Increment services
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 CLIA Kit Customized Service
CLIA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
Citations
- IL17A gene polymorphisms, serum IL-17A and IgE levels, and hepatocellular carcinoma risk in patients with chronic hepatitis B virus infectionPubMed: 23280722
- IL21 and IL21R polymorphisms and their interactive effects on serum IL-21 and IgE levels in patients with chronic hepatitis B virus infectionPubMed: 23354321
- Passive transfer of tumour-derived MDSCs inhibits asthma-related airway inflammationPubmed: 24313384
- Topical Lyogel Containing Corticosteroid Decreases IgE Expression and Enhances the Therapeutic Efficacy Against Atopic EczemaPubMed: 25511806
- Reduced airway microbiota diversity is associated with elevated allergic respiratory inflammationPubMed: 26123423
- The protective role of vitamin D3 in a murine model of asthma via the suppression of TGF-β/Smad signaling and activation of the Nrf2/HO-1 pathway.pubmed:27484042
- Oral administration of Clostridium butyricum CGMCC0313‐1 reduces ovalbumin‐induced allergic airway inflammation in micepubmed:28250847
- Catalpol alleviates ovalbumin-induced asthma in mice: Reduced eosinophil infiltration in the lungpubmed:27992791
- Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in micepubmed:28122397
- Protective effects of astragaloside IV against ovalbumin-induced allergic rhinitis are mediated by T-box protein expressed in T cells/GATA-3 and forkhead box protein 3/retinoic acid-related orphan nuclear receptor γt.pubmed:28586019
- Commensal bacteria aggravate allergic asthma via NLRP3/IL-1β signaling in post-weaning mice10.1016:j.jaut.2018.07.003
- Sub-chronic inhalation of reclaimed water-induced fibrotic lesion in a mouse modelPubmed:29655095
- Investigation of hypersensitivity potential of diacetyl by determining cytokine profilesPubmed:29233007
- Changes in Th1/Th2-producing cytokines during acute exacerbation chronic obstructive pulmonary diseasePubmed:29950127
- Effects of catalpol on bronchial asthma and its relationship with cytokinesPubmed: 30536454
- Adipose Tissue-Derived Mesenchymal Stem Cell Modulates the Immune Response of Allergic Rhinitis in a Rat ModelPubmed: 30781605
- Metabolomics analysis of baicalin on ovalbumin-sensitized allergic rhinitis rats
- Antiallergical Effect of New Combined Nazal Aerodisperse System in the Conditions of Experimental Allergic Rhinitis
- Inhibitory effects of catalpol coordinated with budesonide and their relationship with cytokines and Interleukin-13 expressionPubmed: 31737193
- Animal models for analysis of hypersensitivity reactions to Shuanghuanglian injectionPubmed: 32302806
- Protective effect of Asarum sieboldii essential oil on ovalbumin induced allergic rhinitis in ratPubmed: 32395767
- Synergistic Phage-surfactant Combination Clears IgE-promoted Staphylococcus aureus Aggregation in Vitro and Enhances the Effect in VivoPubmed: 32335278
- Aggravation of Airway Inflammation in RSV-Infected Asthmatic Mice Following Infection-Induced Alteration of Gut Microbiota
- Tissue-Engineered Decellularized Allografts for Anterior Cruciate Ligament Reconstruction
- Biomarkers of Allergic Asthma and their Association with Serum Parameters
- Qingfei oral liquid inhibited autophagy to alleviate inflammation via mTOR signaling pathway in RSV-infected asthmatic mice33706133
- Trace endotoxin in reclaimed water is only one of the risk sources in subchronic inhalation exposure34090073
- Effectiveness of biomarkers and serum parameters in determination allergic asthma and detection of its severity
- Roles Played by the PI3K/Akt/HIF-1α Pathway and IL-17A in the Chinese Subtype of Chronic Sinusitis with Nasal PolypsPubmed:35075349
- Dapagliflozin mitigates ovalbumin-prompted airway inflammatory-oxidative successions and associated bronchospasm in a rat model of allergic asthmaPubmed:35549595