CLIA Kit for Laminin (LN) 
LAM
- UOM
- FOB US$ 588.00 US$ 840.00 US$ 3,780.00 US$ 7,140.00 US$ 58,800.00
- Quantity
Overview
Properties
- Product No.SCA082Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsChemiluminescent immunoassay for Antigen Detection.
Research use only - DownloadInstruction Manual
- Category
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Laminin (LN) and the recovery rates were calculated by comparing the measured value to the expected amount of Laminin (LN) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 85-101 | 90 |
| EDTA plasma(n=5) | 79-93 | 89 |
| heparin plasma(n=5) | 86-99 | 95 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Laminin (LN) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Laminin (LN) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Laminin (LN) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 88-97% | 97-105% | 83-95% | 86-95% |
| EDTA plasma(n=5) | 95-102% | 83-96% | 84-93% | 85-101% |
| heparin plasma(n=5) | 98-105% | 83-101% | 84-101% | 98-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| Substrate A | 1×10mL | Substrate B | 1×2mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
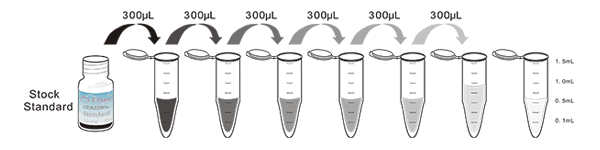
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 100µL Substrate Solution. Incubate 10 minutes at 37°C;
8. Read RLU value immediately.
Test principle
The microplate provided in this kit has been pre-coated with an antibody specific to Laminin (LN). Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated antibody specific to Laminin (LN). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. Then the mixture of substrate A and B is added to generate glow light emission kinetics. Upon plate development, the intensity of the emitted light is proportional to the Laminin (LN) level in the sample or standard.;
Giveaways
Increment services
Citations
- Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton's jellyPubmed: 24739658
- Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented mediaPubmed:Pmc4130592
- Augmented expression of urokinase plasminogen activator and extracellular matrix proteins associates with multiple myeloma progressionPubmed:24807734
- Investigation?of?diagnostic?potentials?of?nine?different?biomarkers?in?endometriosis.Pubmed:24813083
- Comparative Analysis of the Extracellular Matrix Composition in Proliferating and Involuted Infantile HemangiomasPubMed: 26430624
- Modified Citrus Pectin stops progression of liver fibrosis by inhibiting galactin-3 and inducing apoptosis of stellate cellsDoi: Abs
- Clinical significance of serum laminin and typeâIV collagen levels in cutaneous melanoma patientsPubmed:27330797
- Key Matrix Proteins Within the Pancreatic Islet Basement Membrane Are Differentially Digested During Human Islet Isolation.pubmed:27456745
- Modulatory Effects of Chemoradiation on Angiogenic Factors and Laminin in Cervical Cancer: Link with Treatment Responsepubmed:29172262
- Coordination Among Lipid Droplets, Peroxisomes and Mitochondria Regulates Energy Expenditure Through the CIDE-ATGL-PPARα Pathway in AdipocytesPubmed:29986925
- Evaluation of antifibrotic effects of coffee and cocoa extracts in rats with thioacetamide-induced fibrosis10.1007:s00217-018-3119-z
- High amplitude stretching of ATII cells and fibroblasts results in profibrotic effectsPubmed: 31290711
- Fabrication and Evaluation of a Xenogeneic Decellularized Nerve-Derived Material: Preclinical Studies of a New Strategy for Nerve RepairPubmed: 31758411
- Untersuchungen und semiquantitative Symmetrie-Analysen zur Extrazellulärmatrix von Kalbs-Stimmlippen
- CLINICAL SIGNIFICANCE OF LAMININ AND ELASTINE LEVELS IN CHILDREN WITH UNDIFFERENTIATED CONNECTIVE TISSUE DISEASE