ELISA Kit for Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) 
ErbB-1; ErbB1; ER1; ERB-B1; MENA; HER1; Erythroblastic Leukemia Viral(v-Erb-B)oncogene Homolog,Avian; Proto-oncogene c-ErbB-1; Receptor tyrosine-protein kinase erbB-1
- UOM
- FOB US$ 595.00 US$ 850.00 US$ 3,825.00 US$ 7,225.00 US$ 59,500.00
- Quantity
Overview
Properties
- Product No.AEA757Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsEnzyme-linked immunosorbent assay for Antibody Detection.
Research use only - DownloadInstruction Manual
- CategoryCD & Adhesion moleculeTumor immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) and the recovery rates were calculated by comparing the measured value to the expected amount of Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 81-97 | 85 |
| EDTA plasma(n=5) | 86-96 | 91 |
| heparin plasma(n=5) | 82-94 | 91 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Anti-Epidermal Growth Factor Receptor Antibody (Anti-EGFR) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 79-98% | 90-103% | 82-101% | 96-103% |
| EDTA plasma(n=5) | 94-105% | 97-105% | 93-101% | 78-96% |
| heparin plasma(n=5) | 78-97% | 88-95% | 93-105% | 91-101% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 5 times;
5. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
6. Add 50µL Stop Solution. Read at 450nm immediately.

Test principle
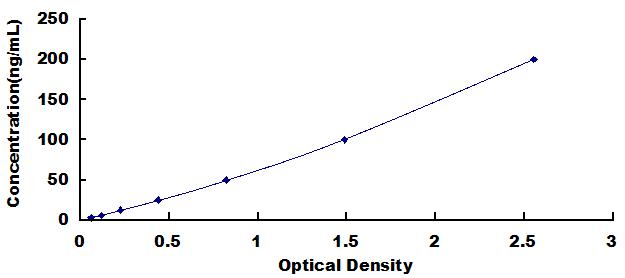
The microtiter plate provided in this kit has been pre-coated with EGFR. Standards or samples are then added to the appropriate microtiter plate wells with a Horseradish Peroxidase (HRP)-conjugated secondary antibody. After TMB substrate solution is added, those wells that contain Anti-EGFR will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of Anti-EGFR in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Giveaways
Increment services
Citations
- Nimotuzumab combined with gemcitabine and cisplatin as second-line chemotherapy for advanced non-small-cell lung cancerWiley: source
- Increased Epidermal Growth Factor Receptor (EGFR) Associated with Hepatocyte Growth Factor (HGF) and Symptom Severity in Children with Autism …Pubmed:25249767
- GC1118, an anti-EGFR antibody with a distinct binding epitope and superior inhibitory activity against high-affinity EGFR ligandsPubMed: 26586721
- LINC01225 promotes occurrence and metastasis of hepatocellular carcinoma in an epidermal growth factor receptor-dependent pathwayPubmed:26938303
- Microfluidic immunosensor based on mesoporous silica platform and CMK-3/poly-acrylamide-co-methacrylate of dihydrolipoic acid modified gold electrode for cancer biomarker detection.pubmed:28335979
- EGFR detection in extracellular vesicles of breast cancer patients through immunosensor based on silica-chitosan nanoplatformDoi: 10.1016/j.talanta.2018.10.016
- Design, synthesis, molecular docking and biological activity evaluation of some novel indole derivatives as potent anticancer active agents and apoptosis inducersPubmed: 30665034
- Novel thienopyrimidine derivatives as dual EGFR and VEGFR-2 inhibitors: design, synthesis, anticancer activity and effect on cell cycle profilePubmed: 30919701
- Design and synthesis of new benzoxazole/benzothiazole-phthalimide hybrids as antitumor-apoptotic agents
- Synthesis and anticancer activity of bis-benzo [d][1, 3] dioxol-5-yl thiourea derivatives with molecular docking studyPubmed: 31288134
- Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters …Pubmed: 31737175
- Patient-derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicinePubmed: 33212364
- Association between Serum Epidermal Growth Factor Receptor and Cyclooxygenase-2 Levels in Patients with Non-small Cell Carcinoma of Lung
- Synthesis, EGFR-TK inhibition and anticancer activity of new quinoxaline derivatives
- Humoral immune response to epidermal growth factor receptor in lung cancer33495907
- Pyrazolo[3,4-d]pyrimidine-based dual EGFR T790M/HER2 inhibitors: Design, synthesis, structure¨Cactivity relationship and biological activity as potential antitumor and anticonvulsant agents33545637
- Design, synthesis, antiproliferative evaluation, and molecular docking study of new quinoxaline derivatives as apoptotic inducers and EGFR inhibitors
- Emitter–Quencher Pair of Single Atomic Co Sites and Monolayer Titanium Carbide MXenes for Luminol Chemiluminescent Reactions34914377
- Discovery of new pyrimido [5, 4-c] quinolines as potential antiproliferative agents with multitarget actions: Rapid synthesis, docking, and ADME studiesPubmed:35219045