ELISA Kit for Interleukin 35 (IL35) 
- UOM
- FOB US$ 490.00 US$ 700.00 US$ 3,150.00 US$ 5,950.00 US$ 49,000.00
- Quantity
Overview
Properties
- Product No.SEC008Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsEnzyme-linked immunosorbent assay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryCytokineTumor immunityInfection immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
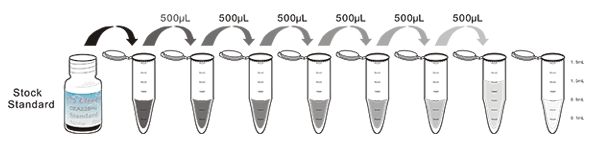
Recovery
Matrices listed below were spiked with certain level of recombinant Interleukin 35 (IL35) and the recovery rates were calculated by comparing the measured value to the expected amount of Interleukin 35 (IL35) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 95-105 | 98 |
| EDTA plasma(n=5) | 84-98 | 93 |
| heparin plasma(n=5) | 86-102 | 90 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Interleukin 35 (IL35) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Interleukin 35 (IL35) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Interleukin 35 (IL35) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 98-105% | 82-101% | 87-105% | 91-98% |
| EDTA plasma(n=5) | 90-101% | 91-105% | 80-96% | 80-105% |
| heparin plasma(n=5) | 78-97% | 94-101% | 78-97% | 87-102% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
Test principle
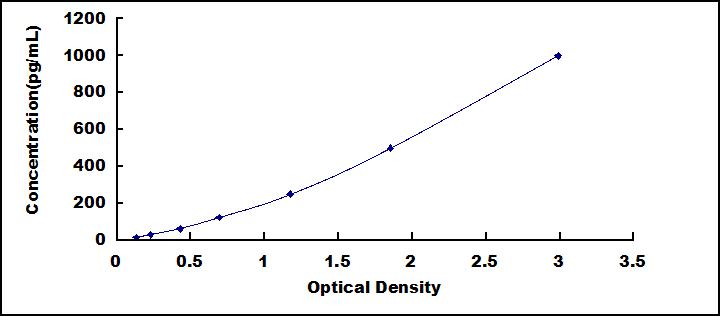
The microplate provided in this kit has been pre-coated with a monoclonal antibody specific to IL12A. Standards or samples are then added to the appropriate microplate wells with a biotin-conjugated monoclonal antibody specific to IL27B. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain IL35, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of IL35 in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Giveaways
Increment services
Citations
- Aberrant expression of Treg-associated cytokine IL-35 along with IL-10 and TGF-β in acute myeloid leukemia.PubMed: 22783403
- Plasma IL-17, IL-35, interferon-γ, SOCS3 and TGF-β levels in pregnant women with preeclampsia, and their relation with severity of diseasePubmed: 24175856
- What is the impact of Th1/Th2 ratio, SOCS3, IL17, and IL35 levels in unexplained infertility?Pubmed: 24368037
- The imbalanced profile and clinical significance of T helper associated cytokines in bone marrow microenvironment of the patients with acute myeloid leukemiaScienceDirect: S0198885913005776
- Gene Expression of Subunits of the IL-12 Family Cytokines in moDCs Derived In Vitro from the Cord Blood of Children of Healthy and Allergic MothersPubmed:24785110
- Function of interleukin?17 and ?35 in the blood of patients with hepatitis B?related liver cirrhosisPubmed:25323532
- The regular distribution and expression pattern of immunosuppressive cytokine IL-35 in mouse uterus during early pregnancyPubmed:25611266
- Wogonin Inhibits Tumor-derived Regulatory Molecules by Suppressing STAT3 Signaling to Promote Tumor ImmunityPubMed: 25962106
- Interleukin 35 Synovial Fluid Levels Are Associated with Disease Activity of Rheumatoid ArthritisPubMed: 26204444
- Interleukin-35 is upregulated in systemic sclerosis and its serum levels are associated with early diseasePubMed: 26231346
- Anti-inflammatory effects of interleukin-35 in acquired aplastic anemiaPubMed: 26282938
- Interleukin-35 Inhibits Endothelial Cell Activation by Suppressing MAPK-AP-1 PathwayPubMed: 26085094
- Interleukin 35 may contribute to the loss of immunological self‐tolerance in patients with primary immune thrombocytopeniaPubMed: 25640666
- New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: A prospective study and a review of the literaturePubmed:26876383
- Increased plasma concentrations of interleukin 35 in patients with coronary artery diseaseJournal:-19
- The clinical utility of serum IL-35 in patients with polymyositis and dermatomyositispubmed:27502600
- Enhanced LPS-induced activation of IL-27 signalling in sarcoidosispubmed:27492538
- Effects of an intravitreal injection of interleukin-35-expressing plasmid on pro-inflammatory andanti-inflammatory cytokines.pubmed:27460435
- IL-35在牙周炎及口腔扁平苔藓中的表达及相关性分析QK:98604X
- Upregulation of Interleukin 35 in Patients With Endometriosis Stimulates Cell Proliferationpubmed:28659009
- IL‑35 may maintain homeostasis of the immune microenvironment in periodontitisissn:2542-3975
- Synoviocytes-derived Interleukin 35 Potentiates B Cell Response in Patients with Osteoarthritis and Rheumatoid Arthritispubmed:29247146
- Human immunodeficiency virus type-1 induces a regulatory B cell-like phenotype in vitropubmed:28713164
- Lower level of IL‑35 and its reduced inhibition in Th17 cells in patients with bone marrow mononuclear cells Coombs test‑positive hemocytopenia.pubmed:29257310
- Evaluation of serum interleukin- 35 level in children with persistent asthma.pubmed:27996283
- Interleukin-35 mitigates the function of murine transplanted islet cells via regulation of Treg/Th17 ratiopubmed:29236782
- Significant association between FOXP3 gene polymorphism and steroid-resistant acute rejection in living donor liver transplantation10.1002/hep4.1052
- Clinical significance of IL-35 expression in the progression of clear cell renal cell carcinoma ISSN:1940-5901
- Interleukin-35 as a predictor of prostate cancer in patients undergoing initial prostate biopsy.pubmed:28761357
- AB0734 Serum il-35 levels in systemic sclerosis and relationship with clinical features10.1136:annrheumdis-2018-eular.2605
- Interleukin-35 as a New Biomarker of Renal Involvement in Lupus Nephritis PatientsPubmed:29576585
- The impact of plasma SOCS3 levels and endometrial leukocytes on unexplained infertilityPubmed:29914245
- Pro-tumoral immune cell alterations in wild type and Shb-deficient mice in response to 4T1 breast carcinomasPubmed:29721156
- Serum Interleukin 35 Levels in Systemic Sclerosis and Relationship With Clinical Features.Pubmed: 30431486
- Interleukin‑35 is associated with the tumorigenesis and progression of prostate cancer
- Plasmacytoid dendritic cells protect against immune-mediated acute liver injury via IL-35Pubmed: 31264967
- CD4+ FOXP3+ T Cells in Rheumatoid Arthritis Bone Marrow Are Partially ImpairedPubmed: 32111105
- Interleukin-35 in idiopathic inflammatory myopathiesPubmed: 33128920
- IL35 predicts prognosis in gastric cancer and is associated with angiogenesis by altering TIMP1, PAI1, and IGFBP1Pubmed: 33064893
- Interleukin-35 promotes progression of prostate cancer and inhibits anti-tumour immunityPubmed: 33041668
- Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brainPubmed: 32375811
- Association of IL-35 expression and gene polymorphisms in rheumatoid arthritis33307515
- Decreased interleukin-35 levels and CD4+ EBI3+ T cells in patients with type 1 diabetes and the effects of the antibody against CD20 (rituximab)33488880
- Anti-inflammatory roles of interleukin-35 in the pathogenesis of Japanese cedar pollinosis34386410
- Pentosan Polysulfate Sodium augments the therapeutic effect of 5-Aminosalicylic Acid in DSS colitis model; the role of IL-35 expressionPubmed:35247859
- Association of three single nucleotide polymorphisms of interleukin-35 for detection and prediction of women with breast cancer in Erbil city
- Analysis of IL-10 and IL-35 in DPP-4 inhibitor-related bullous pemphigoid