Multiplex Assay Kit for Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) 
BMP2A; BMP-2A; Hemochromatosis Modifier
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 472.00 US$ 490.00 US$ 517.00 US$ 553.00 US$ 590.00 US$ 644.00 US$ 726.00 US$ 907.00
- Quantity
Overview
Properties
- Product No.LMA013Po
- Organism SpeciesSus scrofa; Porcine (Pig) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryDevelopmental scienceBone metabolism
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 91-104 | 95 |
| EDTA plasma(n=5) | 82-101 | 98 |
| heparin plasma(n=5) | 94-101 | 97 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Bone Morphogenetic Protein 2 (BMP2) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 87-94% | 86-93% | 96-105% | 98-105% |
| EDTA plasma(n=5) | 85-92% | 90-98% | 93-101% | 97-104% |
| heparin plasma(n=5) | 80-102% | 79-93% | 87-96% | 96-104% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:BMP2) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
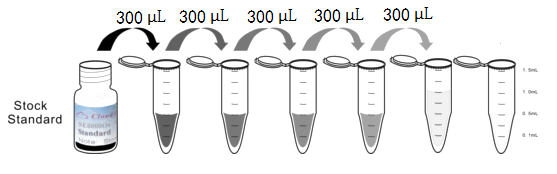
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
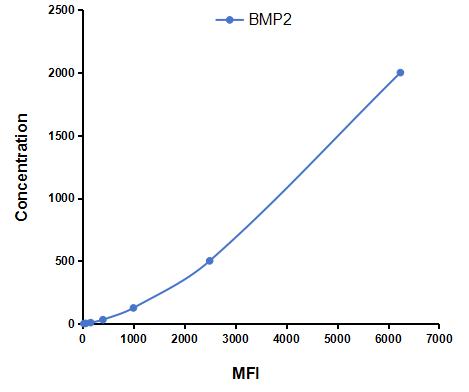
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Chronic exposure to low concentrations of strontium 90 affects bone physiology but not the hematopoietic system in miceWiley: Source
- A Combinatorial Relative Mass Value Evaluation of Endogenous Bioactive Proteins in Three-Dimensional Cultured Nucleus Pulposus Cells of Herniated Intervertebral Discs: Identification of Potential Target Proteins for Gene Therapeutic ApproachesPlosone: Source
- Effect of transplantation of BMP-2-induced bone marrow mesenchymal stem cells on myocardial infarction of rats after reperfusionSource
- PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRα and derepressing BMP-Smad1/5/8 signalingPubmed:25470749
- Negative effect of serotonin–norepinephrine reuptake inhibitor therapy on rat bone tissue after orchidectomyPubMed: 25934570
- Effect of Mirtazapine on Rat Bone Tissue after OrchidectomyPubMed: 25871861
- Titanium with Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling PathwayPubMed: 26681207
- Sustained dual release of placental growth factor-2 and bone morphogenic protein-2 from heparin-based nanocomplexes for direct osteogenesisPubmed:27042064
- EFFECTS OF FLUORIDE ON THE EXPRESSION OF BMP-2 AND SMAD1 IN RAT OSTEOBLASTS IN VITROfiles:p013-022
- Unveiling the interactions among BMPR-2, ALK-1 and 5-HTT genes in the pathophysiology of HAPEPubmed:27196063
- Extracellular Matrix Powder from Cultured Cartilage-Like Tissue as Cell Carriers for Cartilage RepairDOI:10.1039/C7TB00640C
- 3D- Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering.pubmed:29042614
- Demineralized dentin and enamel matrices as suitable substrates for bone regenerationpubmed:28731486
- Peroxisome proliferator‐activated receptor γ plays dual roles on experimental periodontitis in ratsPubmed:29574908
- Effects of Amlodipine on Bone Metabolism in Orchidectomised Spontaneously Hypertensive RatsPubmed:29898457
- BMP-2 and type I collagen preservation in human deciduous teeth after demineralizationPubmed:30045659
- Enhanced osteogenic differentiation of MC3T3-E1 on rhBMP-2 immobilized titanium surface through polymer-mediated electrostatic interaction
- Injectable hydrogels from enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone repair and regeneration
- Development of CaCO 3 microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration
- The dose-time effects of fluoride on the expression and DNA methylation level of the promoter region of BMP-2 and BMP-7 in ratsPubmed: 32004919
- Biofabrication and application of decellularized bone extracellular matrix for effective bone regeneration
- Synergistic Effect of Platelet-rich Fibrin Releasate and Bone Marrow Stem Cells in Preventing Bone Loss in Ovariectomized Mouse
- New design to remove leukocytes from platelet-rich plasma (PRP) based on cell dimension rather than density33842739
- Osteogenic and Angiogenic Potency of VEGF165-Transfected Canine Bone Marrow Mesenchymal Cells Combined with Coral Hydroxyapatite in Vitro34302695
- The WNT/β-catenin signalling pathway induces chondrocyte apoptosis in the cartilage injury caused by T-2 toxin in rats34742779
- Polydopamine-Coated Poly(l-lactide) Nanofibers with Controlled Release of VEGF and BMP-2 as a Regenerative Periosteum34472855
- Efficacy of Total flavonoids of Rhizoma drynariae on the Blood Vessels and the Growth Quality of Bone Graft in the Induced MembranePubmed:35278899