Multiplex Assay Kit for Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) 
CPSD; CLN10; Lysosomal Aspartyl Protease; Ceroid-Lipofuscinosis,Neuronal 10
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 498.00 US$ 517.00 US$ 546.00 US$ 584.00 US$ 623.00 US$ 680.00 US$ 766.00 US$ 958.00
- Quantity
Overview
Properties
- Product No.LMB280Bo
- Organism SpeciesBos taurus; Bovine (Cattle) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryTumor immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 94-101 | 97 |
| EDTA plasma(n=5) | 87-101 | 95 |
| heparin plasma(n=5) | 93-101 | 97 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Cathepsin D (CTSD) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 79-93% | 86-94% | 79-95% | 96-104% |
| EDTA plasma(n=5) | 86-101% | 78-102% | 99-105% | 80-93% |
| heparin plasma(n=5) | 89-102% | 91-99% | 82-95% | 88-99% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:CTSD) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
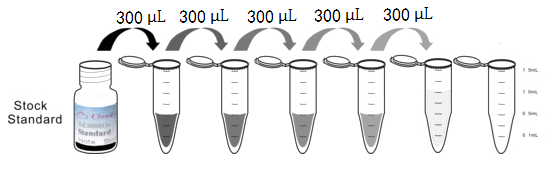
1. Preparation of standards, reagents and samples before the experiment;
2. Add 50μL standard or sample to each well,
add 10μL magnetic beads,and 50μL Detection Reagent A,incubate 60min at 37°C on shaker;
3. Wash plate on magnetic frame for three times;
4. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
5. Wash plate on magnetic frame for three times;
6. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards,Labeled antigen and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest.A competitive inhibition reaction is launched between biotin labeled analytes of interest and unlabeled analytes of interest (Standards or samples) with the pre-coated antibody specific to analytes of interest. Following a wash to remove any unbound substances, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE) is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer. The MFI developed is reverse proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Cathepsin D is released after severe tissue trauma in vivo and is capable of generating C5a in vitroScienceDirect: S0161589011008297
- Plasma Cathepsin D Levels: A Novel Tool to Predict Pediatric Hepatic InflammationPubMed: 25732418
- Plasma cathepsin D correlates with histological classifications of fatty liver disease in adults and responds to interventionpubmed:27922112
- Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: Underlying mechanismspubmed:28061403
- Limited applicability of cathepsin D for the diagnosis and monitoring of non‐alcoholic steatohepatitis
- Plasma cathepsin D activity is negatively associated with hepatic insulin sensitivity in overweight and obese humansPubmed: 31690989
- Iron and Advanced Glycation End Products: Emerging Role of Iron in Androgen Deficiency in ObesityPubmed: 32235809
- LVV-hemorphin-7 (LVV-H7) plays a role in antinociception in a rat model of alcohol-induced pain disorders33253777
- Identification of Cathepsin D as a Plasma Biomarker for Alzheimer's Disease33445607
- Analysis of silymarin-modulating effects against acrylamide-induced cerebellar damage in male rats: Biochemical and pathological markers33965515
- PGRS Domain of Rv0297 of Mycobacterium tuberculosis Is Involved in Modulation of Macrophage Functions to Favor Bacterial Persistence33042856
- Serum CathepsinD in pregnancy: relation with metabolic and inflammatory markers and effects of fish oils and probioticsPubmed:35304048
- A Comparison of Various Chips Used for the Manufacture of Biosensors Applied in Non-Fluidic Array SPRi, Based on the Example of Determination of Cathepsin DPubmed:35049649