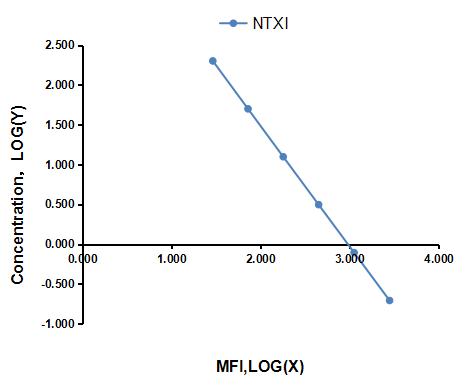
Multiplex Assay Kit for Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) 
NTX-I; NTX1
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 405.00 US$ 420.00 US$ 443.00 US$ 475.00 US$ 506.00 US$ 552.00 US$ 622.00 US$ 778.00
- Quantity
Overview
Properties
- Product No.LMA639Mu
- Organism SpeciesMus musculus (Mouse) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryMetabolic pathwayBone metabolism
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 93-101 | 97 |
| EDTA plasma(n=5) | 80-102 | 95 |
| heparin plasma(n=5) | 95-103 | 99 |
| sodium citrate plasma(n=5) | 79-98 | 95 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Cross Linked N-Telopeptide Of Type I Collagen (NTXI) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 86-98% | 85-96% | 79-92% | 86-99% |
| EDTA plasma(n=5) | 78-91% | 94-102% | 89-101% | 87-105% |
| heparin plasma(n=5) | 87-101% | 93-101% | 84-99% | 86-93% |
| sodium citrate plasma(n=5) | 83-101% | 80-91% | 78-103% | 78-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:NTXI) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 50μL standard or sample to each well,
add 10μL magnetic beads,and 50μL Detection Reagent A,incubate 60min at 37°C on shaker;
3. Wash plate on magnetic frame for three times;
4. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
5. Wash plate on magnetic frame for three times;
6. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
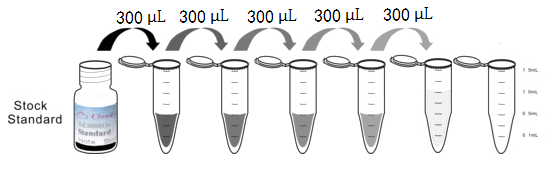
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards,Labeled antigen and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest.A competitive inhibition reaction is launched between biotin labeled analytes of interest and unlabeled analytes of interest (Standards or samples) with the pre-coated antibody specific to analytes of interest. Following a wash to remove any unbound substances, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE) is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer. The MFI developed is reverse proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Dyslipidemic high-fat diet affects adversely bone metabolism in mice associated with impaired antioxidant capacityScienceDirect: S0899900709004717
- Differential mRNA expression profiles in proximal tibia of aged rats in response to ovariectomy and low-Ca dietScienceDirect: S875632820800776X
- Promotion of bone formation by naringin in a titanium particle‐induced diabetic murine calvarial osteolysis modelWiley: Source
- A Mediterranean Diet Enriched with Olive Oil Is Associated with Higher Serum Total Osteocalcin Levels in Elderly Men at High Cardiovascular RiskPubMed: PMC3462931
- Biochemical markers of bone resorption are present in human milk: implications for maternal and neonatal bone metabolismPubmed:25109232
- An NMR Metabolomic Study on the Effect of Alendronate in Ovariectomized MicePubmed:25184758
- Strontium Ranelate Reduces the Fracture Incidence in a Growing Mouse Model of Osteogenesis ImperfectaPubMed: 26679066
- Comparative effects of brown and golden flaxseeds on body composition, inflammation and bone remodelling biomarkers in perimenopausal overweight womenS1756464617301561
- Impact of a chronic smoking habit on the osteo-immunoinflammatory mediators in the peri-implant fluid of clinically healthy dental implants.pubmed:27328151
- Impact of a triclosan‐containing toothpaste during the progression of experimental peri‐implant mucositis: Clinical parameters and local pattern of osteo …Pubmed:29520826
- Triclosan‐containing fluoride toothpaste on clinical parameters and osteo‐inflammatory mediators when applied in a stent during experimental peri‐implant mucositis …Pubmed: 30659666
- AAV-anti-miR-214 prevents collapse of the femoral head in osteonecrosis by regulating osteoblast and osteoclast activitiesPubmed: 31739209
- Material-Dependent Formation and Degradation of Bone Matrix—Comparison of Two CryogelsPubmed: 32517006
- Comparable Effects of Strontium Ranelate and Alendronate Treatment on Fracture Reduction in a Mouse Model of Osteogenesis Imperfecta33506016
- Effects of icariin on the fracture healing in young and old rats and its mechanism34511043
- Gujiansan Ameliorates Avascular Necrosis of the Femoral Head by Regulating Autophagy via the HIF-1α/BNIP3 Pathway34512780