Multiplex Assay Kit for Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) 
HP; Hp2-Alpha; Alpha-2-Macroglobulin; Zonulin
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 449.00 US$ 467.00 US$ 492.00 US$ 527.00 US$ 562.00 US$ 613.00 US$ 691.00 US$ 864.00
- Quantity
Overview
Properties
- Product No.LMA817Ca
- Organism SpeciesCanis familiaris; Canine (Dog) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryInfection immunityHematology
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 81-94 | 91 |
| EDTA plasma(n=5) | 92-101 | 98 |
| heparin plasma(n=5) | 92-101 | 95 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
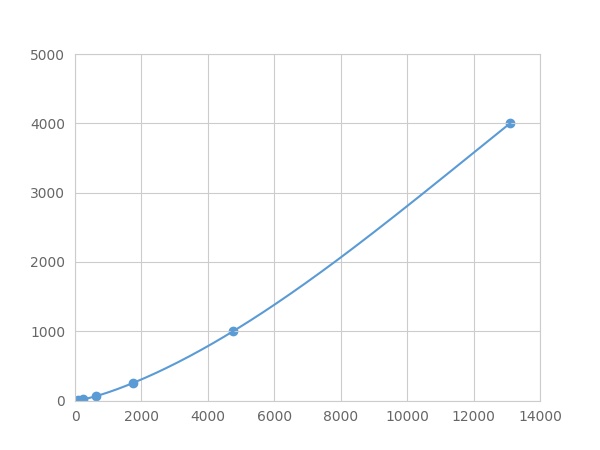
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Haptoglobin (Hpt) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 95-104% | 87-104% | 88-97% | 89-97% |
| EDTA plasma(n=5) | 79-90% | 80-104% | 86-102% | 85-98% |
| heparin plasma(n=5) | 81-88% | 95-102% | 84-95% | 85-92% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:Hpt) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
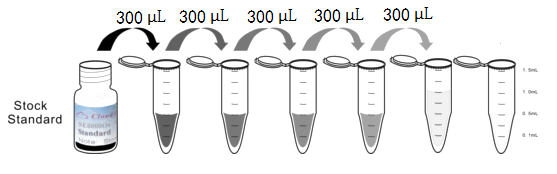
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Circulation levels of acute phase proteins in patients with Takayasu arteritisPubMed: 20100644
- Quantitative trait loci mapping of the mouse plasma proteome (pQTL).NCBI: PMC3567747
- Docosahexaenoic and eicosapentaenoic acid supplementation does not exacerbate oxidative stress or intravascular haemolysis in homozygous sickle cell patients.Pubmed: 24095588
- Gut Microbial Dysbiosis May Predict Diarrhea and Fatigue in Patients Undergoing Pelvic Cancer Radiotherapy: A Pilot StudyPubMed: 25955845
- Application of a new procedure for liquid chromatography/mass spectrometry profiling of plasma amino acid-related metabolites and untargeted shotgun proteomics to identify mechanisms and biomarkers of calcific aortic stenosisS0021967317311858
- High complement levels in astrocyte‐derived exosomes of Alzheimer diseasePubmed:29406582
- Effects of Hippophae rhamnoides L. Leaf and Marc Extract with Reduced Tannin Concentration on the Health and Growth Parameters of Newborn Calves
- Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: growth performance, carcass traits, blood …Pubmed: 30184125
- Urinary Proteomics for the Early Diagnosis of Diabetic Nephropathy in Taiwanese PatientsPubmed: 30486327
- Generation of Pigs Resistant to Highly Pathogenic-Porcine Reproductive and Respiratory Syndrome Virus through Gene Editing of CD163
- Prolyl endopeptidase-degraded low immunoreactive wheat flour attenuates immune responses in Caco-2 intestinal cells and gluten-sensitized BALB/c micePubmed: 31082461
- Ruminally protected and unprotected Saccharomyces cerevisiae fermentation products as alternatives to antibiotics in finishing beef steersPubmed: 31410465
- Deletion of CD163 Exon 7 Confers Resistance to Highly Pathogenic Porcine Reproductive and Respiratory Viruses on Pigs
- Intestinal permeability and small intestine bacterial overgrowth in excess weight adolescentsPubmed: 33089672
- Processing Index of Barley Grain and Dietary Undigested Neutral Detergent Fiber Concentration Affected Chewing Behavior, Ruminal pH and Total Tract Nutrient?¡33474564
- Influence of niacin application on inflammatory parameters, non-esterified fatty acids and functional status of liver in cows during early lactation
- Could serum zonulin be an intestinal permeability marker in diabetes kidney disease?34170951
- The Effect of Feed Supplementation with EM Bokashi® Multimicrobial Probiotic Preparation on Selected Parameters of Sow Colostrum and Milk as Indicators …34596883