Multiplex Assay Kit for Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) 
PLA2G7; PAF-AH; PAFAH; Lp-PLA2; LDL-PLA2; Platelet Activating Factor Acetylhydrolase,Plasma; Phospholipase A2,Group VII; LDL-associated phospholipase A2; Phospholipase A2, Lipoprotein Associated
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 393.00 US$ 408.00 US$ 431.00 US$ 461.00 US$ 491.00 US$ 537.00 US$ 605.00 US$ 756.00
- Quantity
Overview
Properties
- Product No.LMA867Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryEnzyme & KinaseMetabolic pathwayInfection immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 92-99 | 96 |
| EDTA plasma(n=5) | 81-102 | 97 |
| heparin plasma(n=5) | 90-101 | 96 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
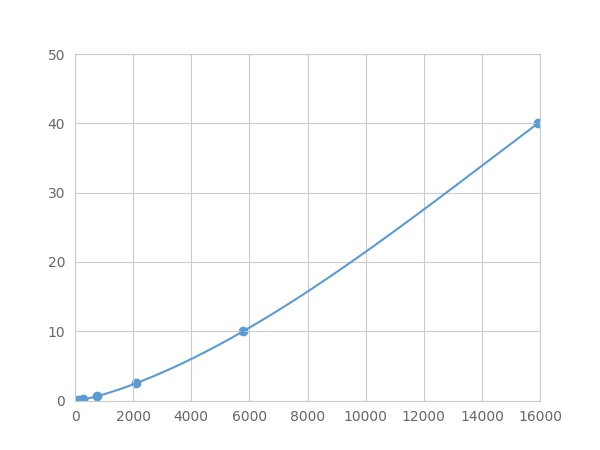
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Phospholipase A2 Group VII (LpPLA2) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 93-101% | 97-105% | 95-102% | 94-101% |
| EDTA plasma(n=5) | 95-103% | 96-103% | 87-94% | 89-97% |
| heparin plasma(n=5) | 98-105% | 98-105% | 82-103% | 91-98% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:LpPLA2) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
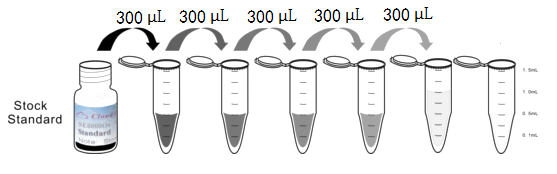
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- The effects of different intensity walking programs on serum blood lipids, high-sensitive C-reactive protein, and lipoprotein-associated phospholipase A2 in premenopausal womenScienceDirect: S0765159710000262
- Association of Serum Lipoprotein-Associated Phospholipase A2 Level with Nonalcoholic Fatty Liver DiseaseWiley: source
- Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over studyPubMed: 22240497
- Effects of Lipid-Lowering Drugs on Irisin in Human Subjects In Vivo and in Human Skeletal Muscle Cells Ex VivoPubMed: PMC3759413
- Influence of Tongfengtai Granules on Inflammatory Factor in Acute Gouty Arthritis Model RatsSource
- Everolimus therapy is associated with reduced lipoprotein-associated phospholipase A2 (Lp-Pla2) activity and oxidative stress in heart transplant recipients.Pubmed: 23958269
- Lipoprotein-Associated Phospholipase A2 Mass Level Is Increased in Elderly Subjects with Type 2 Diabetes MellitusHindawi: 278063
- The Role of Asymmetric Dimethylarginine and Lipoprotein Associated Phospholipase A2 in Children and Adolescents with DyslipidemiaScirp:Source
- In vitro lipid-lowering and fibrinolytic effects of regulatory leucine-containing glyprolines in human bloodSpringer:Source
- Lipoprotein-associated phospholipase A2 and AGEs are associated with cardiovascular risk factors in women with history of gestational diabetes mellitus.Pubmed:24397392
- Lipoprotein-Associated Phospholipase A 2 Mass Level Is Increased in Elderly Subjects with Type 2 Diabetes MellitusPubmed:24818163
- Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetesPubmed:25034387
- Effect of Extended‐Release Niacin on High‐Density Lipoprotein (HDL) Functionality, Lipoprotein Metabolism, and Mediators of Vascular Inflammation in Statin‐Treated PatientsPubMed: 26374297
- VEbscohost
- Effect of Pitavastatin Treatment on ApoB-48 and Lp-PLA2 in Patients with Metabolic Syndrome: Substudy of PROspective Comparative Clinical Study Evaluating the Efficacy and Safety of PITavastatin in Patients with Metabolic Syndromepmc:PMC4803547
- Effect of dipyridamole on myocardial reperfusion injury: A double‐blind randomized controlled trial in patients undergoing elective coronary artery bypass surgeryPubmed:25773594
- Effect of intensive insulin treatment on plasma levels of lipoprotein-associated phospholipase A 2 and secretory phospholipase A 2 in patients with newly …articles:10.1186
- SERUM LIPOPROTEIN-ASSOCIATED PHOSPHOLIPASE A2 IN MALES WITH METABOLIC SYNDROME AND OBSTRUCTIVE SLEEP APNEA.jrnl:1841098
- Biomarker for diagnosis of moyamoya disease:
- Circulating fatty-acid binding-protein 4 levels predict CV events in patients after coronary interventionsPubmed: 32859455
- Can admission lipoprotein-associated phospholipase A2 predict the symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage?