Multiplex Assay Kit for Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) 
P2CP; C-Propeptide Of Type II Procollagen; Procollagen II Carboxy Terminal Propeptide
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 483.00 US$ 502.00 US$ 530.00 US$ 567.00 US$ 604.00 US$ 660.00 US$ 743.00 US$ 929.00
- Quantity
Overview
Properties
- Product No.LMA964Eq
- Organism SpeciesEquus caballus; Equine (Horse) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryInfection immunityBone metabolismRheumatology
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 78-93 | 82 |
| EDTA plasma(n=5) | 93-101 | 96 |
| heparin plasma(n=5) | 93-101 | 96 |
| sodium citrate plasma(n=5) | 92-99 | 95 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Procollagen II C-Terminal Propeptide (PIICP) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 78-96% | 91-98% | 97-105% | 95-102% |
| EDTA plasma(n=5) | 84-101% | 91-99% | 89-102% | 96-104% |
| heparin plasma(n=5) | 93-101% | 87-95% | 85-92% | 89-96% |
| sodium citrate plasma(n=5) | 83-103% | 90-102% | 94-103% | 90-104% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:PIICP) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
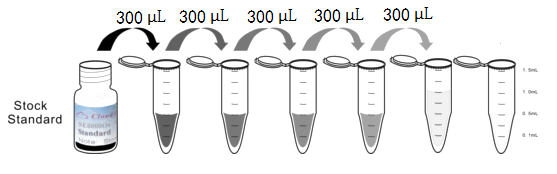
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Evaluation of the effect of N-acetyl-glucosamine administration on biomarkers for cartilage metabolism in healthy individuals without symptoms of arthritis: A …10.3892
- Evaluation of the effect of administering N-acetyl-glucosamine-containing green tea supplement on biomarkers for cartilage metabolism in healthy individuals without symptoms of arthritis: a randomized double-blind placebo-controlled clinical study309
- Effect of N-acetylglucosamine administration on cartilage metabolism and safety in healthy subjects without symptoms of arthritis: A case reportpubmed:28413518
- Evaluation of the effect of salmon nasal proteoglycan on biomarkers for cartilage metabolism in individuals with knee joint discomfort: A randomized double‑blind placebo‑controlled clinical studyetm:14
- Evaluation of the efficacy of Ajuga decumbens extract supplement in individuals with knee discomfort associated with physical activity: A randomized, double‑blind, placebo‑controlled study10.3892/etm.2017.5064
- Evaluation of the chondroprotective action of N‑acetylglucosamine in a rat experimental osteoarthritis pubmed:28912864
- Evaluation of the effect of N-acetyl-glucosamine administration on biomarkers for cartilage metabolism in healthy individuals: a randomized double-blind placebo-controlled clinical studyview/366
- No effects of hyperosmolar culture medium on tissue regeneration by human degenerated nucleus pulposus cells despite upregulation extracellular matrix genesfulltext:2018/03010
- Fibulin-3 and other cartilage metabolism biomarkers in relationship to calprotectin (MRP8/14) and disease activity in rheumatoid arthritis patients treated with …68362.pdf
- サケ鼻軟骨由来プロテオグリカン摂取による関節保護効果
- No Effects of Hyperosmolar Culture Medium on Tissue Regeneration by Human DegeneratedPubmed: 25856264
- Effectiveness of collagen supplementation on pain scores in healthy individuals with self-reported knee pain; A randomized controlled trialPubmed: 31990581
- Bone Morphogenetic Proteins for Nucleus Pulposus RegenerationPubmed: 32295299
- Concerted Actions by PIICP, CTXII, and TNF-¦Á in Patients with Juvenile Idiopathic Arthritis33924892