Multiplex Assay Kit for Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) 
SEPP; SeP; SEP-P1; SELP
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 450.00 US$ 468.00 US$ 494.00 US$ 528.00 US$ 563.00 US$ 615.00 US$ 693.00 US$ 866.00
- Quantity
Overview
Properties
- Product No.LMB809Ra
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryMetabolic pathwayInfection immunity
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 86-99 | 95 |
| EDTA plasma(n=5) | 82-101 | 90 |
| heparin plasma(n=5) | 93-101 | 96 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Selenoprotein P1, Plasma (SEPP1) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 93-101% | 98-105% | 91-99% | 97-104% |
| EDTA plasma(n=5) | 97-104% | 88-95% | 92-101% | 78-105% |
| heparin plasma(n=5) | 92-101% | 88-101% | 79-101% | 99-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:SEPP1) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
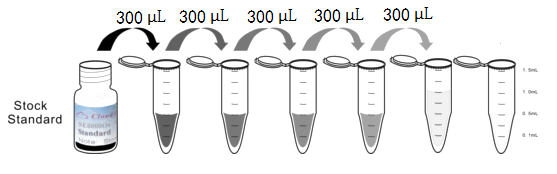
Test principle
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards, and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest. After washing away any unbound substances, a biotinylated antibody cocktail specific to the analytes of interest is added to each well. Following a wash to remove any unbound biotinylated antibody, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated detection antibodies, is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer.The MFI developed is proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- Serum Selenoprotein P Levels in Patients with Type 2 Diabetes and Prediabetes: Implications for Insulin Resistance, Inflammation, and AtherosclerosisPubMed: 21677040
- Increased Selenoprotein P Levels in Subjects with Visceral Obesity and Nonalcoholic Fatty Liver DiseasePubMed: 23439771
- Trace elements, heavy metals and vitamins in Egyptian school children with iron deficiency anemiaMetapress:Source
- Selenium and its relationship with selenoprotein P and glutathione peroxidase in children and adolescents with Hashimoto's thyroiditis and hypothyroidismPubMed: 26854239
- Selenoprotein P is elevated in individuals with obesity, but is not independently associated withinsulin resistance.pubmed:27524654
- Acute Overfeeding Does Not Alter Liver or Adipose Tissue-Derived Cytokines in HealthyHumans.pubmed:27832637
- DNA damage and oxidative stress response to selenium yeast in the non-smoking individuals: ashort-term supplementation trial with respect to GPX1 and SEPP1 polymorphism.pubmed:26658762
- Evaluation of the Relationship Between Insulin Resistance and Selenoprotein P in Patients with Polycystic Ovary SyndromeDOI: 10.14744/scie.2017.87597
- Serum selenium and selenoprotein-P levels in autoimmune thyroid dieases patients in a select center a transversal studyDOI: 10.1590/2359-3997000000309
- Selenium, selenoproteins and selenometabolites in mothers and babies at the time of birthpubmed:28534447
- Biomarkers of selenium status and antioxidant effect in workers occupationally exposed to mercuryPubmed:29895371
- Comparison of Human Selenoprotein P Determinants in Serum between Our Original Methods and Commercially Available KitsPubmed:29709922
- Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitusPubmed: 30470912
- Selenium Levels in Community Dwellers with Type 2 Diabetes MellitusPubmed: 30725267
- Can hepatokines be regarded as novel non-invasive serum biomarkers of intrahepatic lipid content in obese children?Pubmed: 30921653
- Acute swimming exercise, but not exposure to moderate hypoxic conditions reduces circulating selenoprotein P levels in short-term, high-fat diet-fed ratsPubmed: 31236416
- Selenium and selenoprotein P in nonalcoholic fatty liver diseasePubmed: 31493247
- Soil Selenium Concentration and Residents Daily Dietary Intake in a Selenosis Area: A Preliminary Study in Yutangba Village, Enshi City, ChinaPubmed: 32909074
- Higher Serum Selenoprotein P Level as a Novel Inductor of Metabolic Complications in PsoriasisPubmed: 32605214
- Circulating hepassocin level in patients with stable angina is associated with fatty liver and renal function
- Selenium–GPX4 axis protects follicular helper T cells from ferroptosis34413521
- Selenoprotein P-1 (SEPP1) as An Early Biomarker of Acute Kidney Injury in Patients Undergoing Cardiopulmonary Bypass