Multiplex Assay Kit for Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) 
(Note: Up to 8-plex in one testing reaction)
- UOM
- FOB US$ 427.00 US$ 443.00 US$ 468.00 US$ 501.00 US$ 534.00 US$ 583.00 US$ 657.00 US$ 821.00
- Quantity
Overview
Properties
- Product No.LMA393Ra
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- ApplicationsFLIA Kit for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryEndocrinologyNeuro scienceHormone metabolism
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 87-102 | 97 |
| EDTA plasma(n=5) | 94-103 | 97 |
| heparin plasma(n=5) | 96-103 | 101 |
| sodium citrate plasma(n=5) | 85-95 | 92 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Substance P (SP) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 79-97% | 94-103% | 91-98% | 90-98% |
| EDTA plasma(n=5) | 82-103% | 83-93% | 91-99% | 96-104% |
| heparin plasma(n=5) | 83-98% | 97-105% | 92-105% | 87-94% |
| sodium citrate plasma(n=5) | 97-105% | 91-99% | 92-101% | 78-105% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:SP) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 50μL standard or sample to each well,
add 10μL magnetic beads,and 50μL Detection Reagent A,incubate 60min at 37°C on shaker;
3. Wash plate on magnetic frame for three times;
4. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
5. Wash plate on magnetic frame for three times;
6. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
Test principle
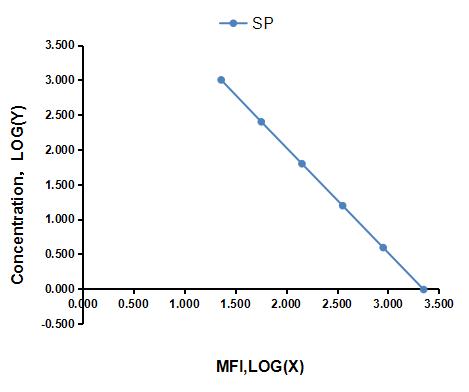
Analyte-specific antibodies are pre-coated onto color-coded microparticles. Microparticles, standards,Labeled antigen and samples are pipetted into wells and the immobilized antibodies bind the analytes of interest.A competitive inhibition reaction is launched between biotin labeled analytes of interest and unlabeled analytes of interest (Standards or samples) with the pre-coated antibody specific to analytes of interest. Following a wash to remove any unbound substances, Streptavidin-Phycoerythrin conjugate (Streptavidin-PE) is added to each well. A final wash removes unbound Streptavidin-PE and the microparticles are resuspended in buffer and read using the Luminex or Bio-Plex analyzer. The MFI developed is reverse proportional to the concentration of analytes of interest in the sample.
Giveaways
Increment services
Citations
- A Potential Role for Substance P and Interleukin-6 in the Cerebrospinal Fluid of Cavalier King Charles Spaniels with Neuropathic PainPubmed: 23659719
- Oral curcumin has anti-arthritic efficacy through somatostatin generationPubMed: 25836921
- Positive enhancement of Lactobacillus fermentum HY01 on intestinal movements of mice having constipation10.1007/s13765-017-0327-3
- Direct activation of tachykinin receptors within baroreflex afferent pathway and neurocontrol of blood pressure regulationPubmed:29900692
- Serum Levels of Neuropeptides in Cows with Left Abomasal DisplacementPubmed: 30562932
- Effects of nanoparticles on Neuroinflammation in a Mouse Model of AsthmaPubmed: 31542455
- Hejie Zhitong prescription promotes sleep and inhibits nociceptive transmission-associated neurotransmitter activity in a rodent migraine modelPubmed: 33014123
- Changes of substance P, NGF and CGRP salivary levels among patients undergoing physical therapy
- Evaluation of the causes affecting the development of pruritus in patients with peritoneal dialysis34213714
- Surfactant protein C is associated with perineuronal nets and shows age-dependent changes of brain content and hippocampal deposits in wildtype and 3xTg mice34626771