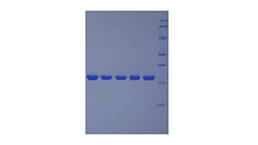
Active Peptidylglycine Alpha Amidating Monooxygenase (PAM) 
PAL; PHM; Peptidylamidoglycolate lyase; Peptidyl-Alpha-hydroxyglycine Alpha-amidating Lyase; Peptidylglycine Alpha-Hydroxylating Monooxygenase
- UOM
- FOB US$ 374.00 US$ 935.00 US$ 1,870.00 US$ 5,610.00 US$ 14,025.00
- Quantity
Overview
Properties
- Product No.APC744Hu61
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsCell culture; Activity Assays.
Research use only - DownloadInstruction Manual
- CategoryEnzyme & Kinase
- Buffer FormulationPBS, pH7.4, containing 5% Trehalose.
- Traits Freeze-dried powder, Purity > 95%
- Isoelectric Point6.1
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Activity test
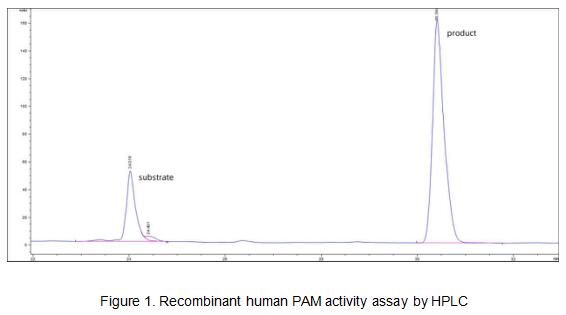
Peptidyl-glycine alpha-amidating monooxygenase (PAM) is an enzyme that is required for the biosynthesis of many signaling peptides. It has two enzymatically active domains with catalytic activities-peptidylglycine alpha-hydroxylating monooxygenase (PHM) and peptidyl-alpha-hydroxyglycine alpha-amidating lyase (PAL). These catalytic domains work sequentially to catalyze neuroendocrine peptides to active alpha-amidated products. A typical activity assay was using Dns-Tyr-Val-Gly as substrate, thus the activity of recombinant human PAM was measured by its ability to hydrolyze Dns-Tyr-Val-Gly to Dns-Tyr-Val-NH2. The reaction was performed in 100 mM MES/KOH pH 6.0, 30 mM KI, 30 mM KCl, 1 uM cupric sulfate, 100 ug/ml catalase (APC418Hu05), 1% (v/v) ethanol, 0.001% (v/v) Triton X-100 and 10 mM ascorbate. 250 ul 0.7 mM substrate of Dns-Tyr-Val-Gly was added with 250 ul various concentrations of recombinant human PAM (1 ug/ml,5 ug/ml, 10 ug/ml and 20 ug/ml). Incubated at 37℃ for 30min, the reaction was stopped by addition 6% (v/v) TCA. The product and substrate was detected by RP-HPLC with UV-detection at 280 nm, the analyses were performed at 25℃ employing a Agilent ZORBAX Poroshell SB C18 column (9.4×250 mm, 5 μm), the flow rate was 1 ml/min. The mobile phase consisted of 100 mM sodium acetate (pH 6.5) and 30 min linear gradient of 10-90% acetonitrile. At 30-35 min, the mobile phase consisted of 90% acetonitrile and 10% sodium acetate. The result was shown in Figure 1. As the Figure 1 shows, the substrate have been hydrolyzed to Dns-Tyr-Val-NH2 after incubating with recombinant human PAM. The retention time of Dns-Tyr-Val-Gly and Dns-Tyr-Val-NH2 is 24.088 and 30.421 respectively (Figure 2). The specific activity of recombinant human PAM is >6600 pmol/min/µg.
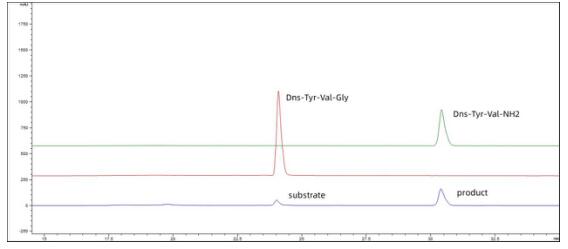
Figure 2. The reaction product compared with standard Dns-Tyr-Val-Gly and Dns-Tyr-Val-NH2.
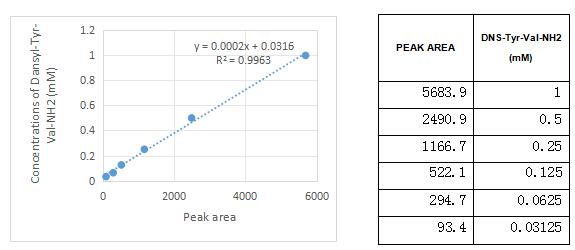
Figure 3. The sandard curve of Dns-Tyr-Val-NH2
Usage
Reconstitute in 10mM PBS (pH7.4) to a concentration of 0.1-1.0 mg/mL. Do not vortex.
Storage
Avoid repeated freeze/thaw cycles. Store at 2-8°C for one month. Aliquot and store at -80°C for 12 months.
Stability
The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37°C for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition.
Increment services
-
 BCA Protein Quantification Kit
BCA Protein Quantification Kit
-
 Molecular Mass Marker for Protein
Molecular Mass Marker for Protein
-
 Monoclonal Antibody Customized Service
Monoclonal Antibody Customized Service
-
 Polyclonal Antibody Customized Service
Polyclonal Antibody Customized Service
-
 Protein Activity Test Experiment Service
Protein Activity Test Experiment Service
-
 Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
-
 Buffer
Buffer
-
 Lentivirus Packaging Experiment Service
Lentivirus Packaging Experiment Service
-
 Adenovirus Packaging Experiment Service
Adenovirus Packaging Experiment Service
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Spike RBD Protein (S-RBD)
Spike RBD Protein (S-RBD)
-
 Protein G
Protein G
-
 Protein A
Protein A