ELISA Kit for Surfactant Protein B (SP-B) 
SFTPB; PSPB; SFTB3; SFTP3; SPB; Surfactant Associated Protein B; Pulmonary Surfactant Protein B; 18 kDa pulmonary-surfactant protein; 6 kDa protein
- UOM
- FOB US$ 466.00 US$ 665.00 US$ 2,993.00 US$ 5,653.00 US$ 46,550.00
- Quantity
Overview
Properties
- Product No.SEB622Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- ApplicationsEnzyme-linked immunosorbent assay for Antigen Detection.
Research use only - DownloadInstruction Manual
- CategoryTumor immunityInfection immunityPulmonology
Sign into your account
Share a new citation as an author
Upload your experimental result
Review

Contact us
Please fill in the blank.
Recovery
Matrices listed below were spiked with certain level of recombinant Surfactant Protein B (SP-B) and the recovery rates were calculated by comparing the measured value to the expected amount of Surfactant Protein B (SP-B) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 99-105 | 102 |
| EDTA plasma(n=5) | 85-104 | 94 |
| heparin plasma(n=5) | 82-105 | 94 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Surfactant Protein B (SP-B) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Surfactant Protein B (SP-B) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Surfactant Protein B (SP-B) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 79-98% | 79-103% | 97-105% | 80-102% |
| EDTA plasma(n=5) | 98-105% | 88-95% | 95-102% | 81-102% |
| heparin plasma(n=5) | 78-94% | 89-96% | 82-104% | 95-104% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| Pre-coated, ready to use 96-well strip plate | 1 | Plate sealer for 96 wells | 4 |
| Standard | 2 | Standard Diluent | 1×20mL |
| Detection Reagent A | 1×120µL | Assay Diluent A | 1×12mL |
| Detection Reagent B | 1×120µL | Assay Diluent B | 1×12mL |
| TMB Substrate | 1×9mL | Stop Solution | 1×6mL |
| Wash Buffer (30 × concentrate) | 1×20mL | Instruction manual | 1 |
Assay procedure summary
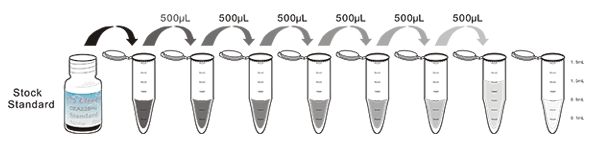
1. Prepare all reagents, samples and standards;
2. Add 100µL standard or sample to each well. Incubate 1 hours at 37°C;
3. Aspirate and add 100µL prepared Detection Reagent A. Incubate 1 hour at 37°C;
4. Aspirate and wash 3 times;
5. Add 100µL prepared Detection Reagent B. Incubate 30 minutes at 37°C;
6. Aspirate and wash 5 times;
7. Add 90µL Substrate Solution. Incubate 10-20 minutes at 37°C;
8. Add 50µL Stop Solution. Read at 450nm immediately.
Test principle
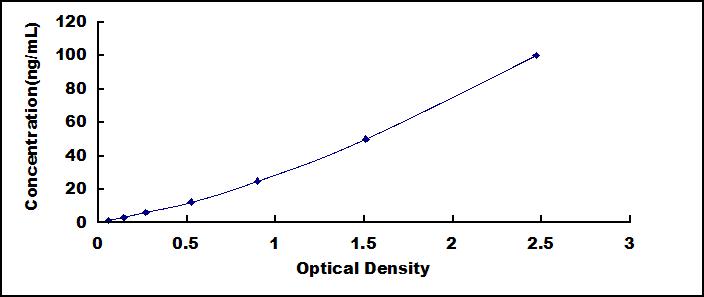
The test principle applied in this kit is Sandwich enzyme immunoassay. The microtiter plate provided in this kit has been pre-coated with an antibody specific to Surfactant Protein B (SP-B). Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated antibody specific to Surfactant Protein B (SP-B). Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain Surfactant Protein B (SP-B), biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450nm ± 10nm. The concentration of Surfactant Protein B (SP-B) in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Giveaways
Increment services
-
 Single-component Reagents of Assay Kit
Single-component Reagents of Assay Kit
-
 Lysis Buffer Specific for ELISA / CLIA
Lysis Buffer Specific for ELISA / CLIA
-
 Quality Control of Kit
Quality Control of Kit
-
 ELISA Kit Customized Service
ELISA Kit Customized Service
-
 Disease Model Customized Service
Disease Model Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 TGFB1 Activation Reagent
TGFB1 Activation Reagent
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Streptavidin
Streptavidin
-
 Fast blue Protein Stain solution
Fast blue Protein Stain solution
-
 Single-component Reagents of FLIA Kit
Single-component Reagents of FLIA Kit
-
 Streptavidin-Agarose Beads
Streptavidin-Agarose Beads
Citations
- Kinetics of plasma SPB and RAGE during mechanical ventilation in patients undergoing major vascular surgeryPubMed: 21736957
- Detection of surfactant proteins A, B, C, and D in human nasal mucosa and their regulation in chronic rhinosinusitis with polypsPubMed: 23406594
- Surfactant protein B and RAGE increases in the plasma during cardiopulmonary bypass: a pilot studyPubMed: 20650982
- Staphylococcus aureus and Pseudomonas aeruginosa Express and Secrete Human Surfactant ProteinsPubMed: PMC3551896
- The Detection of Surfactant Proteins A, B, C and D in the Human Brain and Their Regulation in Cerebral Infarction, Autoimmune Conditions and Infections of the CNSPubMed: PMC3787032
- Lung Cancer Signatures in Plasma Based on Proteome Profiling of Mouse Tumor ModelsPubMed: PMC3406925
- Acute high-altitude exposure reduces lung diffusion: Data from the HIGHCARE Alps projectPubmed: 23619193
- Surfactant-Derived Proteins as Markers of Alveolar Membrane Damage in Heart FailurePubmed:25514679
- Nachweis und Charakterisierung des Oberfl?chenproteins PLUNC (Palate, Lung and Nasal Clone Protein) an der Augenoberfl?che und Bedeutung für das Trockene AugeOpus4:Source
- Plasma immature form of surfactant protein type B correlates with prognosis in patients with chronic heart failure. A pilot single-center prospective studyPubMed: 26310985
- Serum Levels of Surfactant Proteins in Patients with Combined Pulmonary Fibrosis and Emphysema (CPFE)Pubmed:27337142
- The Cerebral Surfactant System and Its Alteration in HydrocephalicConditions.pubmed:27656877
- Correlations of Ventricular Enlargement with Rheologically Active SurfactantProteins in Cerebrospinal Fluid.pubmed:28101052
- Correlations of Ventricular Enlargement with Rheologically Active Surfactant Proteins in Cerebrospinal FluidPMC5209370
- Diving and pulmonary physiology: surfactant binding protein, lung fluid and cardiopulmonary test changes in professional diverspubmed:28467885
- Surfactant Protein B Suppresses Lung Cancer Progression by Inhibiting Secretory Phospholipase A2 Activity and Arachidonic Acid Productionpubmed:28743125
- Chronic lung injury and impaired pulmonary function in a mouse model of acid ceramidase deficiency.pubmed:29167126
- Effects and molecular mechanisms of intrauterine infection/inflammation on lung developmentPubmed:29747649
- Surfactant proteins changes after acute hemodynamic improvement in patients with advanced chronic heart failure treated with LevosimendanPubmed:29548887
- Serum Surfactant Protein Levels in Patients Admitted to the Hospital with Acute COPD ExacerbationPubmed:29445934
- Generation of an alveolar epithelial type II cell line from induced pluripotent stem cellsPubmed: 30211653
- Culture of human alveolar epithelial type II cells by sproutingPubmed: 30340591
Citations
-
 s**********b@**dizin.uni-leipzig.deAugust 24, 2018Elevated Surfactant Protein Levels and Increased Flow of Cerebrospinal Fluid in Cranial Magnetic Resonance Imaging.
s**********b@**dizin.uni-leipzig.deAugust 24, 2018Elevated Surfactant Protein Levels and Increased Flow of Cerebrospinal Fluid in Cranial Magnetic Resonance Imaging.Recommend products