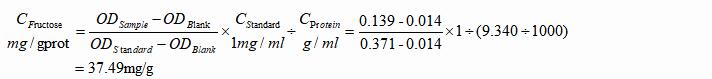Fructose Assay Kit (A085)
Instruction manual
FOR IN VITRO AND RESEARCH USE ONLY
NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES
First Edition (Revised on April, 2016)
[ INTENDED USE ]
The kit is a colorimetric method for the in vitro quantitative measurement of Fructose in juice, honey, semen, tissue, etc..
Fructose in vivo is secreted by seminal vesicles and is the main saccharide components within semen as a major source of energy for sperm. Thus the fructose concentration significantly relates to the motility of sperm. The concentration will directly influence the motility of sperm and is a factor to judge the motility.
Fructose is the sweetest of all naturally occurring carbohydrates and is 1.73 times as sweet as sucrose. The fructose can be found in juice or honey and is a main factor to judge such products’ quality. The method establishment of fructose measurement signify the quality control of juice and honey.
[ REAGENTS AND MATERIALS PROVIDED ]
Reagents | Quantity(50T-48S) | Reagents | Quantity(50T-48S) |
Reagent 1 | 2×75ml | Reagent 2 | 3 |
Instruction manual | 1 |
[ MATERIALS REQUIRED BUT NOT SUPPLIED ]
1. An UV-spectrophotometer capable of measuring absorbance at 285nm
2. Thermostatic water bath or air bath capable of controlling temperature at 100℃
3. Test tube
4. Micropipets and tips
5. Vortex mixer
6. A source of pure water (preferably double distilled water and double distilled water)
[ STORAGE OF THE KITS ]
1. Reagent I: Substrate solution. Preserved at 4℃ for 6 months.
2. Reagent II: Fructose Standard. Preserved at 4℃ for 6 months.
[ REAGENT PREPARATION ]
Reagent 2 Solution Preparation: Dissolve each bottle of powder with distilled water to 10 ml final volume. The final concentration is 1mg/ml.
[ SAMPLE PREPARATION ]
1. Juice Samples
Take the freshly prepared juice and centrifuge at 3,500 rpm for 10 min. Extract the supernatant for further measurement.
2. Honey Samples
Samples should be diluted with DDW to 1000 times of its initial volume for measurement.
3. Semen Samples
Pretreat the sample through liquefaction process and centrifuge the mixture at 2,500 rpm for 15 min. Extract the supernatant for measurement.
4. Tissue Samples
Weigh the tissue precisely and add physiological saline with the ratio 1 g tissue to 4 ml saline. Homogenize the mixture and centrifuge the homogenate at 2,500 rpm for 10 min.
Note that turbidity may appear for the high concentration and under such circumstance, centrifugation is recommended to be done with 4,000 rpm with the period of 10 min.
[ ASSAY PROCEDURE ]
Operation table:
Compositions (ml) | Blank | Standard | Sample |
Double Distilled Water (DDW) | 0.05 | ||
Reagent II Solution | 0.05 | ||
Sample Solution | 0.05 | ||
Reagent I | 3 | 3 | 3 |
Mix thoroughly and boil the mixture in a water bath for 8 min. Cool the mixture with water and zero the cuvettes at 285 nm with 1 cm path length using DDW. Record the optical density (OD) value of each tube at 285 nm.
[ TEST PRINCIPLE ]
Fructose reacts with the substrate solution (reagent I) and the product has a maximum absorbance value at 285 nm. The absorbance can be measured by spectrophotometer and thus the amount of fructose can be measured.
[ CALCULATION OF RESULTS ]
1. Juice Samples
a. Formula

Note: CoD represents the coefficient of dilution in the pretreatment process.
b. Example
Freshly prepared cantaloupe juice was centrifuged and the supernatant was diluted with DDW to 100 times of its initial volume. The OD values were 0.014, 0.371 and 0.129 respectively.
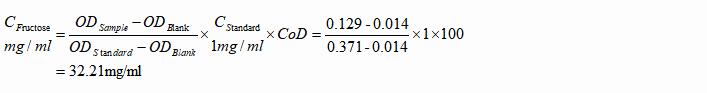
2. Honey Samples
a. Formula

b. Example
Honey sample was diluted with DDW to 1000 times of its initial volume and measured. The OD values were 0.014, 0.371 and 0.372 respectively.
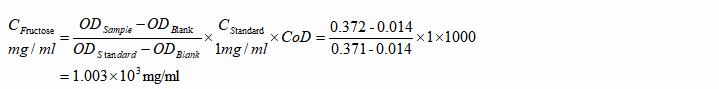
3. Semen Samples
a. Formula

b. Example
Dilute the pre-treated semen sample with DDW to 5 times of its initial volume. Then the sample was measured with OD values 0.014, 0.371 and 0.342 respectively.
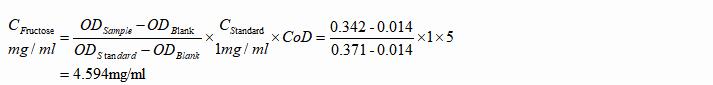
4. Tissue Samples
a. Formula

b. Example
Rat testicle tissue was pre-treated and measured. The OD values were 0.014, 0.371 and 0.139 respectively. The protein concentration of the homogenate was measured to be 9.340mg/ml.