Eukaryotic Programmed Cell Death Protein 1 (PD1) 
CD279; PDCD1; SLEB2; HPD1P
- UOM
- FOB US$ 240.00 US$ 600.00 US$ 1,200.00 US$ 3,600.00 US$ 9,000.00
- Quantity
Overview
Properties
- Product No.EPA751Hu61
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
-
Applications
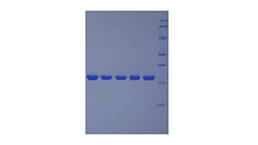
Positive Control; Immunogen; SDS-PAGE; WB.
If bio-activity of the protein is needed, please check active protein.
Research use only - DownloadInstruction Manual
- CategoryApoptosisImmune moleculeEndocrinologyAutoimmunity
- Source Eukaryotic expression, Host 293F Cell
- Endotoxin Level<1.0EU per 1µg (determined by the LAL method)
- Subcellular LocationMembrane
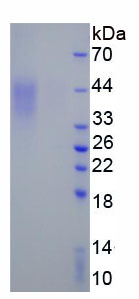
- Molecular Mass 18.4kDa, Accurate 36-45kDa(Analysis of differences refer to the manual)
- Residues & TagsPro21~Val170 with N-terminal His Tag
- Buffer FormulationPBS, pH7.4, containing 5% Trehalose.
- Traits Freeze-dried powder, Purity > 95%
- Isoelectric Point8.7
Share your citation
Upload your experimental result
Review
Leave a message
Loading...
Sign into your account
Share a new citation as an author
Upload your experimental result
Review
Please attach serial No. on instruction manual


Contact us
Please fill in the blank.
Name*
Organization
Address
E-mail address*
Telephone
Inquiry*
Verification code*
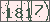
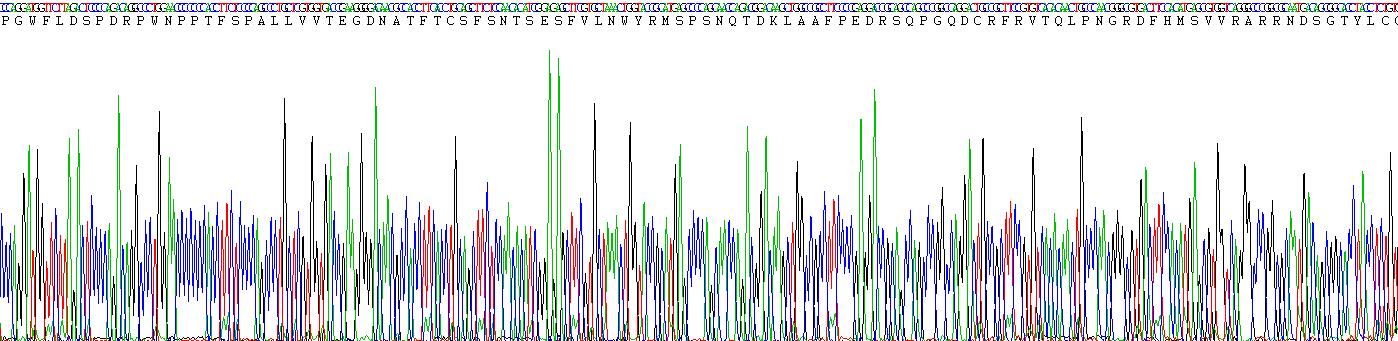
Sequence

Usage
Reconstitute in 10mM PBS (pH7.4) to a concentration of 0.1-1.0 mg/mL. Do not vortex.
Storage
Avoid repeated freeze/thaw cycles. Store at 2-8°C for one month. Aliquot and store at -80°C for 12 months.
Stability
The thermal stability is described by the loss rate. The loss rate was determined by accelerated thermal degradation test, that is, incubate the protein at 37°C for 48h, and no obvious degradation and precipitation were observed. The loss rate is less than 5% within the expiration date under appropriate storage condition.
Increment services
-
 BCA Protein Quantification Kit
BCA Protein Quantification Kit
-
 Protein Labeling Customized Service
Protein Labeling Customized Service
-
 Molecular Mass Marker for Protein
Molecular Mass Marker for Protein
-
 Monoclonal Antibody Customized Service
Monoclonal Antibody Customized Service
-
 Polyclonal Antibody Customized Service
Polyclonal Antibody Customized Service
-
 Protein Activity Test Experiment Service
Protein Activity Test Experiment Service
-
 Immunoprecipitation (IP) Experiment Service
Immunoprecipitation (IP) Experiment Service
-
 Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
Electrophoretic Mobility Shift Assay (EMSA) Experiment Service
-
 Buffer
Buffer
-
 Lentivirus Packaging Experiment Service
Lentivirus Packaging Experiment Service
-
 Adenovirus Packaging Experiment Service
Adenovirus Packaging Experiment Service
-
 Real Time PCR Experimental Service
Real Time PCR Experimental Service
-
 Spike RBD Protein (S-RBD)
Spike RBD Protein (S-RBD)
-
 Protein G
Protein G
-
 Protein A
Protein A
Citations
- Circulating soluble programmed death-1 levels may differentiate immune-tolerant phase from other phases and hepatocellular carcinoma from other clinical diseases in chronic hepatitis B virus infection.pubmed:28545019
- Потенциальные биомаркеры эффективности терапии ниволумабом при метастатическом почечно-клеточном раке:
- АНАЛИЗ РЕЗУЛЬТАТОВ ЛЕЧЕНИЯ БОЛЬНЫХ МЕТАСТАТИЧЕСКИМ РАКОМ ПОЧКИ, ПОЛУЧАВШИХ АНТИ-PD-1‑ТЕРАПИЮ В РАМКАХ ПРОГРАММЫ …
- Design and Synthesis of A PD-1 Binding Peptide and Evaluation of Its Anti-Tumor ActivityPubmed: 30699956
- Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated …Pubmed: 30807610
- Predictive Value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-Small Cell Lung Cancer: A Case-Control StudyPubmed: 32085544