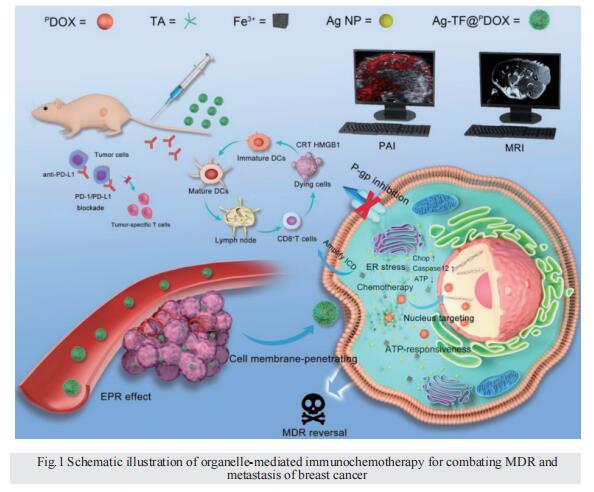
Combating multidrug resistance and metastasis of breast cancer by endoplasmic reticulum stress and cell-nucleus penetration enhanced immunochemotherapy

On 21 March 2022, Jianli Ren, Department of Ultrasound and Chongqing Key Laboratory of Ultrasound Molecular Imaging, the Second Affiliated Hospital of Chongqing Medical University, P. R. China, and his team published a paper tittled “Combating multidrug resistance and metastasis of breast cancer by endoplasmic reticulum stress and cell-nucleus penetration enhanced immunochemotherapy” in Theranostics, which presented a nanocomposite that can restrict the growth of drug-resistant breast tumors and activates a strong immune response as well.

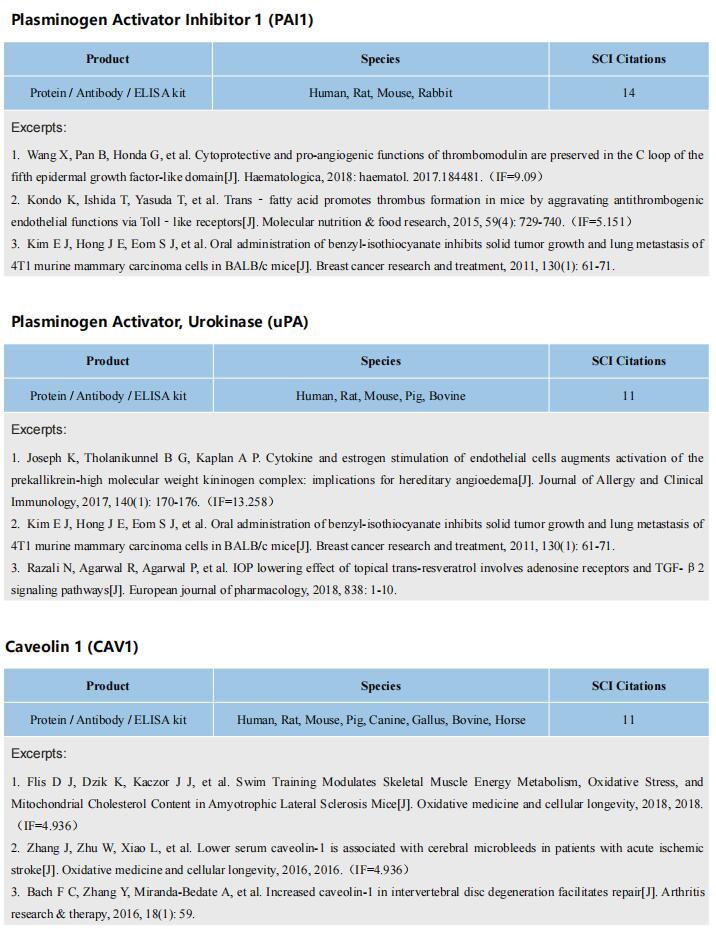
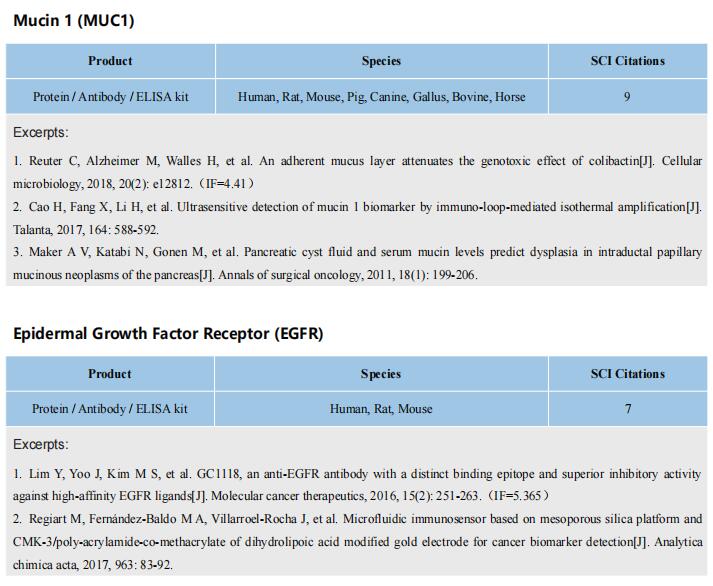
The kits [ELISA Kit for Tumor Necrosis Factor Alpha (TNFa), SEA133Mu; ELISA Kit for Interferon Gamma (IFNg), SEA049Mu; ELISA Kit for Interleukin 6 (IL6), SEA079Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.



Rationale: Multidrug resistance (MDR) and metastasis of breast cancer remain major hurdles in clinical anticancer therapy. The unsatisfactory outcome is largely due to insufficient cytotoxicity of chemotherapeutic agents and limited immunogenic cell death (ICD). On the other hand, efflux proteins, especially P-glycoprotein (P-gp), can recognize and promote the efflux of drugs from tumor cells.
Methods: In this study, silver nanoparticles (Ag NPs) and peptide- functionalized doxorubicin (PDOX) were used to prepare a theranostic nanocomposite (Ag-TF@PDOX), which induced organelle-mediated immunochemotherapy and drug efflux protein inhibition in drug-resistant breast cancer cells (MCF-7/ADR) via a strategy based on endoplasmic reticulum (ER) stress and cell-nucleus penetration.
Results: The silver nanoparticle-triggered persistent activation of ER stress synergizes with chemotherapy to enhance cytotoxicity and stimulate the ICD effect. It has the potential to enhance chemosensitivity by downregulating of P-gp expression due to the increased production of ATP-consuming chaperones. In addition, the novel peptide (CB5005), which not only penetrates the cell membrane but also has a nuclear localization sequence, is conjugated to DOX to improve both cellular internalization and intranuclear accumulation. Moreover, surface TA-Fe3+ engineering endows the nanocomposite with ATP-responsive disassembly and ATP depletion properties to improve biocompatibility and decrease ATP-dependent drug efflux. Ag-TF@PDOX has potential as a dual-mode (PAI/MRI) contrast-enhanced agent for realizing theranostic guidance.
Conclusion: This theranostic nanocomposite greatly restricts the growth of drug-resistant breast tumors and activates a strong immune response as well, providing an opportunity for the development of therapeutics that reverse tumor MDR and metastasis at the subcellular level.
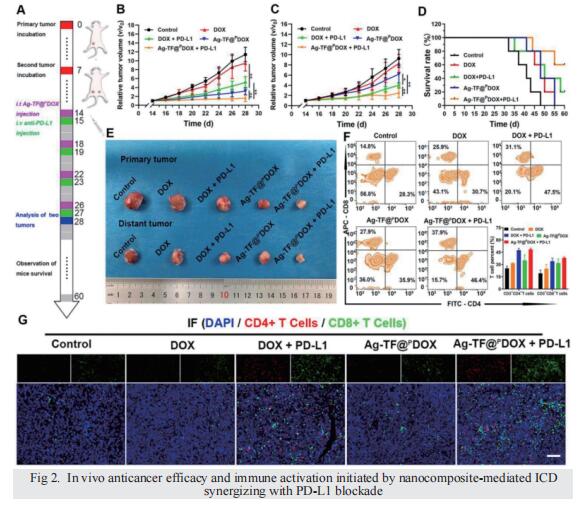
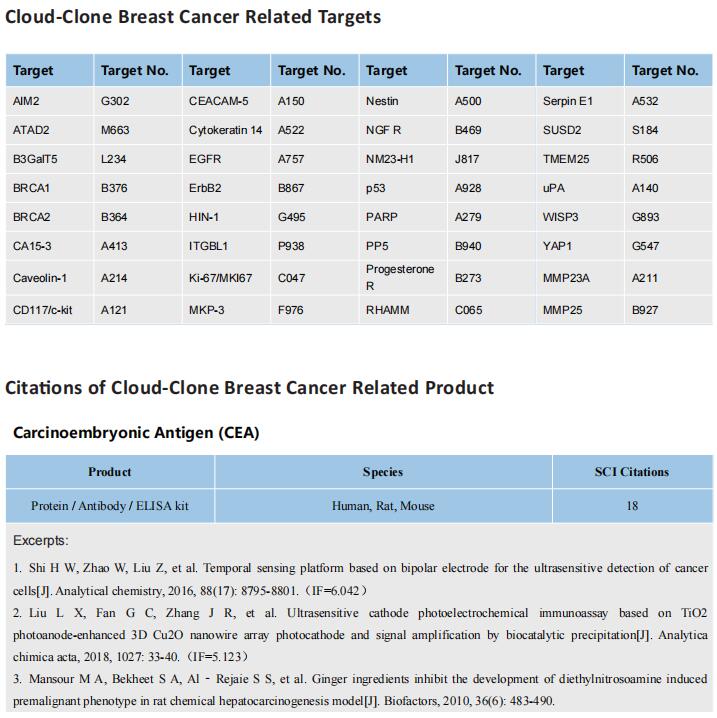
Breast cancer is one of the most common cancers in women. The incidence of breast cancer ranks in the forefront of all kinds of cancers. The peak incidence of breast cancer is between 45 and 60 years old. In recent years, the incidence of breast cancer is younger. With the development of the breast cancer study, more and more prognostic markers have been found. Some of them have been applied in clinical practice as the basis of molecular typing of breast cancer, for example, estrogen receptor (ER), progesterone receptor (PR) and proto-oncogene Her2 are negative markers of triple-negative breast cancer, the prognosis is poor. There are more than a dozen breast cancer molecular typing based on biomarkers, which can detect the occurrence or recurrence of breast cancer.