Imbalance leads to disease, regulation for treatment: Focusing on the disease code and therapeutic breakthroughs of TCR signal transduction
Imbalance leads to disease, regulation for treatment: Focusing on the disease code and therapeutic breakthroughs of TCR signal transduction
1. Introduction to T-cell Receptors
T cells are the key mediators for generating effective cell-mediated adaptive immune responses. The development of T cells in the thymus involves T cell receptor (TCR) signaling. Cells carrying TCR with high affinity for their own peptide-MHC complexes undergo apoptosis, while cells carrying TCR with low affinity survive and differentiate into mature T cells. These mature T cells leave the thymus and enter peripheral lymphoid organs. During pathogenic infections, they are exposed to exogenous peptides presented by MHC molecules of antigen-presenting cells (APCs). When TCR binds to antigenic peptides, T cells are activated, undergo clonal expansion and differentiation to exert their effector functions. Therefore, TCR signaling is of great significance for effective T cell development, activation and immune tolerance, and its signal dysregulation may lead to immune anergy or autoimmunity.
1.1 Structure of the TCR Complex
The TCR complex is a transmembrane protein complex formed by the non-covalently binding of the TCR heterodimer (αβ or γδ chain) responsible for specific antigen recognition and the CD3 complex (composed of γε, δε and ζζ dimers) responsible for signal transduction. The variable region of TCR recognizes the antigenic peptide-MHC complex, while the intracellular region of the CD3 subunit contains the immune receptor tyrosine activating motif (ITAM), which can transmit the recognition signal into the interior of the cell, thereby initiating the activation of T cells and immune responses.
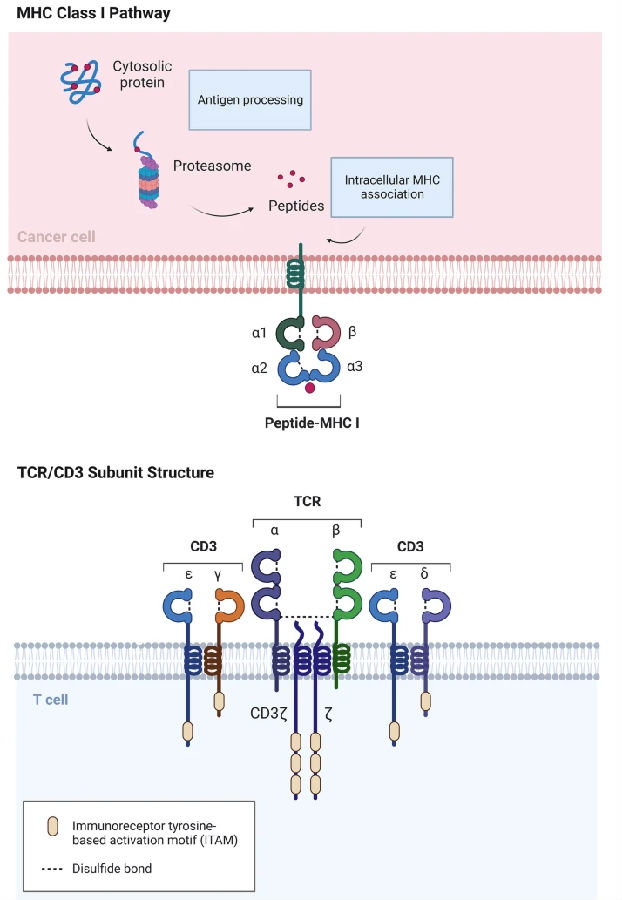
Fig.1 Schematic diagram of TCR/CD3 and peptide MHC structures
(The figure is sourced from Exp Hematol Oncol[1])
1.2 TCR Activation and Proximal Signal Transduction
The activation of TCR begins with its specific binding to the MHC-peptide complex on the surface of APC. This binding induces conformational changes in the TCR-CD3 complex, activating the Src family kinase Lck linked to CD4 or CD8 co-receptors. The activated Lck immediately phosphorylates the tyrosine residues on the intracellular region ITAM of the CD3 chain, providing anchoring sites for downstream signaling molecules. Phosphorylated ITAM recruits and activates another key kinase, ZAP70, which in turn phosphorylates multiple adaptor proteins, such as linker for activation of T-cell (LAT) and SH2 domain containing leukocyte protein of 76kDa (SLP76), promoting their assembly into a large signaling complex. This proximal signal event rapidly amplifies the initial signal near the cell membrane and ultimately conducts it through multiple pathways such as phospholipase Cγ (PLCγ) activation, calcium ion mobilization, and Ras/MAPK, driving the activation, proliferation, and effector function initiation of T cells.
1.3 TCR Distal Signal Transduction
After TCR activation, distal signal transduction refers to a series of downstream signal cascade reactions triggered by the initial membrane proximal signal. These signals are transmitted to the nucleus through the main pathways: on the one hand, PLCγ hydrolyzes phosphatidylinositol 4, 5-diphosphate (PIP2) to produce inositol 1,4, 5-triphosphate (IP3) and diacylglycerol (DAG). IP3 mediates the continuous increase of intracellular calcium ion concentration, activates calcineurin, dephosphorylates the transcription factor NFAT and enters the nucleus; On the other hand, DAG works in synergy with proteins such as RasGRP to activate the Ras/MAPK pathway and protein kinase Cθ (PKCθ), which in turn activates the transcription factor NF-κB. These signals eventually converge in the cell nucleus and drive the clonal proliferation, differentiation and functional execution of T cells by inducing the expression of specific genes, thus completing the immune response.
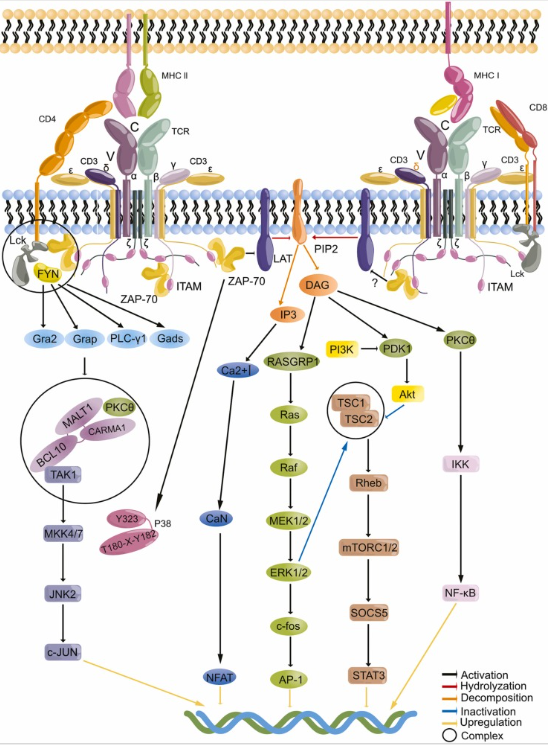
Fig.2 TCR signal transduction
(The figure is sourced from Pharmacol Res[2])
2. Research on TCR signal transduction and cancer
TCR signaling is the core mechanism by which T cells recognize and eliminate abnormal cells, and it plays a "double-edged sword" role in the occurrence and development of cancer. Cutaneous squamous cell carcinoma (cSCC) is mainly caused by UV-mediated carcinogenesis, and β -human tumor virus (HPV) is regarded as a cofactor in maintaining cSCC. Individuals with impaired T-cell response are more susceptible to the carcinogenic effects mediated by β-HPV. Restoring TCR signal integrity through allogeneic hematopoietic cell transplantation can improve HPV-related invasive and refractory cSCC[3]. Targeted degradation of T-cell kinase (ITK) enhances the sensitivity of T-cell lymphoma cells to cytotoxic chemotherapy by regulating the TCR signaling intensity and blocking the activation of NF-kB/GATA-3 signaling[4]. TCR pathway genes are overexpressed in T-cell acute lymphoblastic leukemia (T-ALL) and mature T-cell carcinoma. Blocking ZAP70 can inhibit the growth and survival of T-ALL[5]. Butyrate metabolites in the gut microbiota promote the anti-tumor efficacy of anti-PD-1 immunotherapy by regulating the TCR signaling of cytotoxic CD8+ T cells[6]. Incorporating the cytoplasmic domain of CD3ε into the second-generation chimeric antigen receptor (CAR) can enhance the anti-tumor activity of CAR-T cells[7]. During the differentiation of exhausted CD8+ T cells, the TCR library was expanded by fine-tuning the TCR signaling intensity mediated by caspase recruitment domain family member 11 (CARD11), thereby reactivating the anti-tumor function[8]. These studies have highlighted the core value of targeting the TCR signaling pathway in cancer immunotherapy.
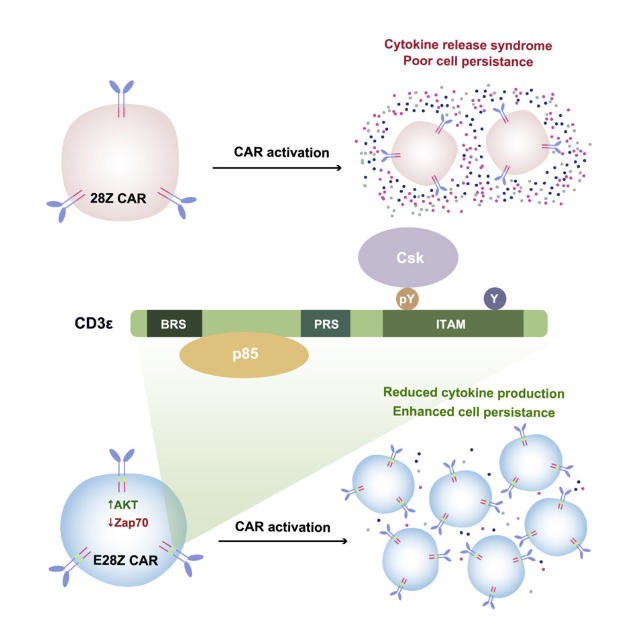
Fig.3 Incorporating CD3ε into 28Z CAR promotes anti-tumor function
(The figure is sourced from Cell[7])
3. Research on TCR signal transduction and autoimmune diseases
The dysregulation of the TCR signaling pathway is one of the key links that break autoimmune tolerance and drive the occurrence of autoimmune pathology. TCR signaling induces STAT3 phosphorylation through Lck/Fyn and collaborates with cytokine signaling to drive TH17 cell differentiation[9]. Selective inhibition of STAT3 phosphorylation induced by TCR stimulation significantly inhibits TH17 differentiation and alleviates TH17 cell-related autoimmune diseases. The intracellular domain of lymphocyte activating gene 3 (LAG-3) can form liquid-liquid phase separated condensates with CD3ε, disrupting the binding of CD3ε to Lck and ultimately weakening the signal transduction of TCR[10]. The use of bispecific antibodies that simultaneously target the LAG-3 and TCR complex on the surface of T cells can achieve LAG-3 dependent T cell inhibition and effectively alleviate autoimmune symptoms in mouse models. A signal transduction connector protein 1 (STAP-1) -derived peptide iSP1 can inhibit the interaction between STAP-1 and Lck, suppress TCR-mediated signal transduction, and inhibit T cell proliferation in humans and mice[11]. In addition, iSP1 slows down the progression of experimental autoimmune encephalomyelitis by inhibiting the infiltration of Th1 and Th17 cells. A protein called Raftlin can regulate TCR signaling. Its deficiency can induce a reduction in the production of T-cell-dependent antibodies, alleviating experimental autoimmune encephalomyelitis[12]. Therefore, in-depth research on the TCR signal transduction mechanism is of great significance for understanding the pathogenesis of autoimmune diseases and developing targeted treatment strategies.
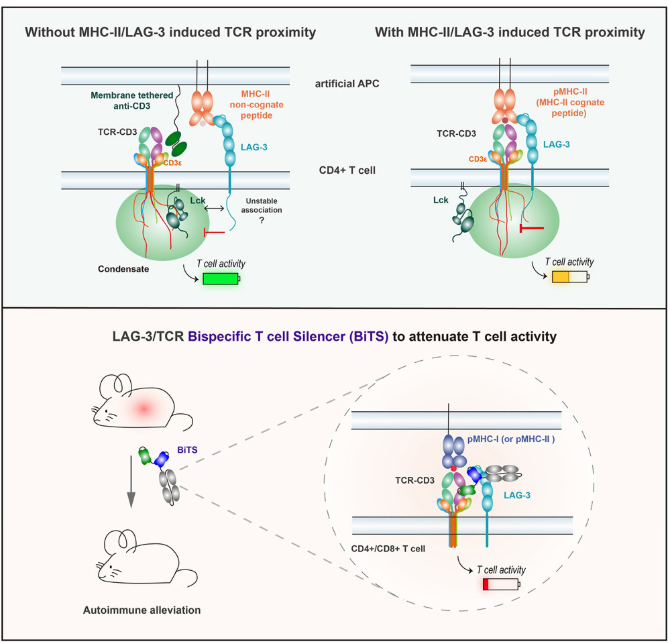
Fig.4 The LAG-3/TCR bispecific antibody can effectively inhibit autoimmunity
(The figure is sourced from Cell[10])
4. Research on TCR signal transduction and cardiovascular diseases
TCR signaling is at the core of T cell activation and function, and it plays a significant role in the occurrence and development of cardiovascular diseases. In the single-cell RNA sequencing analysis of CD8+ T cells from 30 patients with coronary artery disease (CAD) and 30 control groups, it was found that the TCR signaling pathway was significantly enriched in CAD subjects[13]. Compared with the control group, the CD8+ T cells in the blood of CAD cases that respond to atherosclerotic antigens are more significant. ATP-binding cassette transporter A1 (ABCA1) is crucial for T-cell cholesterol homeostasis. The absence of ABCA1 in T cells impairs TCR signaling, inhibits the survival rate, proliferation rate, differentiation and function of T cells, and thereby provides atherosclerotic protection in vivo[14]. Asparagine endopeptidase (LGMN) plays a key role in regulating the function of CD4+ T cells and the progression of atherosclerosis. LGMN deficiency can damage the TCR signaling pathway, inhibit the activation, survival and proliferation of CD4+ T cells, and thereby reduce T cell accumulation in plaque lesions[15]. AX-024 is a molecule that inhibits the interaction between TCR and the noncatalytic region of tyrosine kinase (Nck), and can suppress antigen-specific T-cell responses. Studies have shown that AX-024 effectively inhibits the activation of antigen-specific and non-specific T cells in the hemorrhagic brain, as well as the activation of peripheral antigen-specific T cells in a mouse model of intracerebral hemorrhage (ICH)[16]. AX-024 reduces molecular and cellular neuroinflammation, alleviates short-term and long-term brain damage, and improves functional recovery after ICH. Therefore, targeting the TCR signaling pathway has become a potential therapeutic strategy for regulating the immune inflammatory process of cardiovascular diseases.
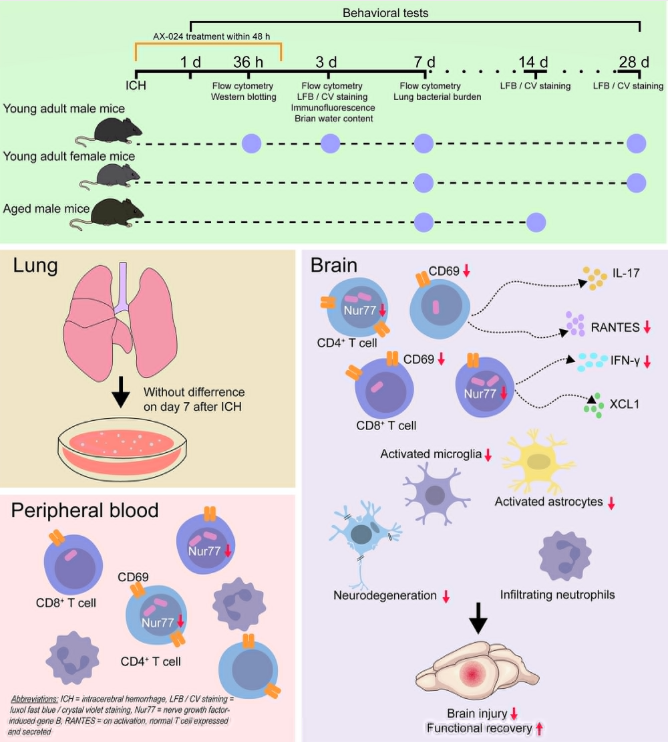
Fig.5 AX-024 improves the outcome of intracerebral hemorrhage
(The figure is sourced from Stroke[16])
5. Research on TCR signal transduction and allergic diseases
TCR signal transduction is a key initiating link in the occurrence of allergic diseases. CD8 epidermal resident memory T (T+RM) cells play a significant role in the pathogenesis of various inflammatory skin diseases. Studies have shown that CD8 T+RM cells can develop in the skin in the absence of homologous antigens, but TCR signaling is necessary for the persistence of allergen-specific CD8 T+RM cells in the skin[17]. SPA0355 inhibits the activation, proliferation and differentiation of CD4 T cells by regulating TCR signal transduction and cytokine-induced JAK/STAT, regulates Th1 and Th2 responses, and exerts a protective effect in a mouse model of ovoprotein-induced allergic airway inflammation[18]. Microarray analysis of gene expression in CD4+ cells of patients with seasonal allergic rhinitis (SAR) revealed that 25 out of 38 TCR pathway genes were expressed differently[19]. Further analysis indicates that haplotypes covering the main part of the ITK coding sequence are risk factors for SAR. Il-2-induced ITK is crucial for TCR signaling and plays a key role in the pathogenesis of asthma. A novel small molecule ITK inhibitor, C-161, was discovered through compound screening. C-161 can prevent the release of pro-inflammatory cytokines induced by TCR, as well as the activation and differentiation of Th2 and Th17 cells in a dose-dependent manner, and alleviate the progression of asthma by reducing inflammatory cell infiltration and the production of mucus and IgE[20]. Therefore, TCR signaling is a potential therapeutic target for allergic immune responses.
Cloud-Clone supports scientific research and provides relevant detection reagent products for a wide range of scientific researchers. The core product numbers of the relevant targets are as follows:
Target | core product No. | Target | core product No. | Target | core product No. |
Bcl10 | C326 | CTLA4 | B230 | MAP2K7 | D560 |
CAM | A640 | DAG | C038 | NFAT | A504 |
CaN | B323 | ERK1 | B357 | NFATC1 | L941 |
CD28 | A652 | ERK2 | A930 | NFATC2 | L942 |
CD3d | B872 | GADS | H967 | NFkB | B824 |
CD3e | D117 | Grb2 | C514 | PDK1 | C718 |
CD3g | D118 | IKKA | K407 | PIK3Ca | J830 |
CD3z | A865 | IL2 | A073 | PIK3Cb | J829 |
CD4 | B167 | ITK | H774 | PIP3 | G856 |
CD45 | B030 | LAT | A270 | PLCg1 | A269 |
CD8a | B099 | MAP2K1 | D559 | SHP1 | D589 |
CD8b | Q127 | MAP2K2 | D562 | SLP76 | B205 |
c-Jun | B292 | MAP2K4 | D564 | zAP70 | P541 |
For more scientific research reagents, please visit the official website of Cloud-Clone:http://www.cloud-clone.com/
References
[1]Shao W, Yao Y, Yang L, et al. Novel insights into TCR-T cell therapy in solid neoplasms: optimizing adoptive immunotherapy. Exp Hematol Oncol. 2024;13(1):37.
[2]Liu Y, Chen S, Liu S, et al. T-cell receptor signaling modulated by the co-receptors: Potential targets for stroke treatment. Pharmacol Res. 2023;192:106797.
[3]Ye P, Bergerson JRE, Brownell I, et al. Resolution of Squamous-Cell Carcinoma by Restoring T-Cell Receptor Signaling. N Engl J Med. 2025;393(5):469-478.
[4]Jiang B, Weinstock DM, Donovan KA, et al. ITK degradation to block T cell receptor signaling and overcome therapeutic resistance in T cell lymphomas. Cell Chem Biol. 2023;30(4):383-393.e6.
[5]Suske T, Sorger H, Manhart G, et al. Hyperactive STAT5 hijacks T cell receptor signaling and drives immature T cell acute lymphoblastic leukemia. J Clin Invest. 2024;134(8):e168536.
[6]Zhu X, Li K, Liu G, et al. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes. 2023;15(2):2249143.
[7]Wu W, Zhou Q, Masubuchi T, et al. Multiple Signaling Roles of CD3ε and Its Application in CAR-T Cell Therapy. Cell. 2020;182(4):855-871.e23.
[8]Hu Y, Zhao Q, Qin Y, et al. CARD11 signaling regulates CD8+ T cell tumoricidal function. Nat Immunol. 2025;26(7):1113-1126.
[9]Qin Z, Wang R, Hou P, et al. TCR signaling induces STAT3 phosphorylation to promote TH17 cell differentiation. J Exp Med. 2024;221(3):e20230683.
[10]Du J, Chen H, You J, et al. Proximity between LAG-3 and the T cell receptor guides suppression of T cell activation and autoimmunity. Cell. 2025;188(15):4025-4042.e20.
[11]Sasaki Y, Kagohashi K, Kawahara S, et al. STAP-1-derived peptide suppresses TCR-mediated T cell activation and ameliorates immune diseases by inhibiting STAP-1-LCK binding. Immunohorizons. 2025;9(6):vlaf015.
[12]Saeki K, Fukuyama S, Ayada T, et al. A major lipid raft protein raftlin modulates T cell receptor signaling and enhances th17-mediated autoimmune responses. J Immunol. 2009;182(10):5929-5937.
[13]Iqneibi S, Saigusa R, Khan A, et al. Single cell transcriptomics reveals recent CD8T cell receptor signaling in patients with coronary artery disease. Front Immunol. 2023;14:1239148.
[14]Zhao Y, Zhang L, Liu L, et al. Specific Loss of ABCA1 (ATP-Binding Cassette Transporter A1) Suppresses TCR (T-Cell Receptor) Signaling and Provides Protection Against Atherosclerosis. Arterioscler Thromb Vasc Biol. 2022;42(12):e311-e326.
[15]Xiang X, Zhang F, Nie L, et al. Legumain deficiency halts atherogenesis by modulating T cell receptor signaling. Aging Cell. 2025;24(2):e14391.
[16]Chen S, Fu P, Rastegar-Kashkooli Y, et al. AX-024 Inhibits Antigen-Specific T-Cell Response and Improves Intracerebral Hemorrhage Outcomes in Mice. Stroke. 2025;56(5):1253-1265.
[17]Funch AB, Weber JF, Mraz V, et al. CD8+ Skin-Resident Memory T Cells Require TCR Signaling for their Persistence in a Mouse Model of Allergic Contact Dermatitis. J Invest Dermatol. 2026;146(1):165-174.e3.
[18]Jang HY, Jeon R, Kang KW, et al. SPA0355 suppresses T-cell responses and reduces airway inflammation in mice. Eur J Pharmacol. 2014;745:19-28.
[19]Benson M, Mobini R, Barrenäs F, et al. A haplotype in the inducible T-cell tyrosine kinase is a risk factor for seasonal allergic rhinitis. Allergy. 2009;64(9):1286-1291.
[20]Guo Z, Ye F, Zhang Y, et al. A Novel Small Molecule ITK Inhibitor Suppresses Th2/Th17 Differentiation and Attenuates Airway Inflammation in a Mouse Model of HDM-Induced Asthma. Immunology. 2025;176(3):349-362.


