New discovery of COPD disease mechanism
Chronic obstructive pulmonary disease (COPD) is now the third most common cause of death in the world. COPD is characterized by progressive airflow obstruction and continuously aggravated airflow limitation with an abnormal and persistent inflammatory response of the lungs or airways to cigarette smoking or harmful gases and particles. Tobacco smoking and second-hand exposure to cigarette smoke (CS), are the major causes of COPD in developed countries, but air pollution and other exposures are also important.
New discovery of COPD disease mechanism
There is no cure for COPD, and current treatments, including physiotherapeutic and pharmacologic interventions, aim to mitigate symptoms and acute exacerbations. However, they do not modify the long-term decline in lung function in patients with COPD. Development of therapies is hampered by the lack of understanding of the mechanisms that drive disease pathogenesis and progression. Recently, a number of high score papers have reported new insights into COPD disease as a potential approach for targeted treatment of COPD.
1. Suv4-20h1-mediated epigenetic regulation prevents COPD
Epigenetic mechanisms may be involved in the development of COPD abnormalities.To identify pathology-associated epigenetic alteration in diseased lung tissues, Thomas Braun of Max Planck Institute for Heart and Lung Research, and his team screened a cohort of human patients with PH and COPD for changes of histone modifications by immunofluorescence staining.[1] They discovered a strong reduction of the histone modifications of H4K20me2/3 in human patients with COPD. Loss of Suv4-20h1 caused a COPD-like phenotype in mice including the formation of perivascular tertiary lymphoid tissue and goblet cell hyperplasia, hyperproliferation of smooth muscle cells/myofibroblasts, impaired alveolarization and maturation defects of the microvasculature(Fig.1). Mechanistically, SUV4-20H1 binds directly to the 5′-upstream regulatory element of the superoxide dismutase 3 (SOD3) gene to repress its expression. Increased levels of the extracellular SOD3 enzyme increases hydrogen peroxide concentrations, causing vascular defects and impairing alveolarization. The study will facilitate the understanding of pathogenic events causing COPD and aid the development of epigenetic drugs for the treatment of COPD.
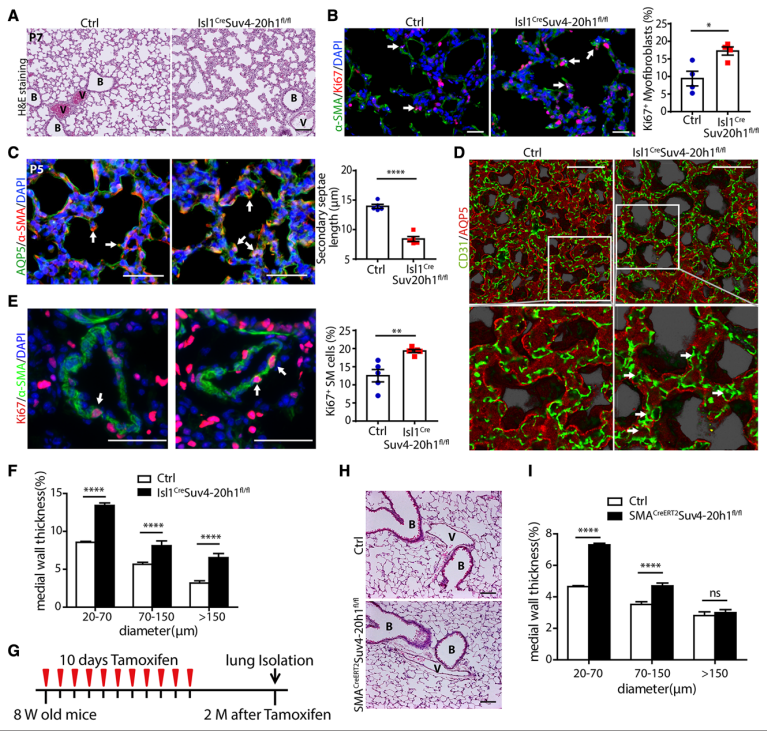
Fig.1 Inactivation of Suv4-20h1 leads to impaired alveolarization and vascular remodeling during postnatal lung development
2. A microRNA-21-mediated SATB1/S100A9/NF-κB axis promotes COPD
Roles for microRNAs (miRNAs) in health and disease are established, and increasing clinical and experimental evidence implicates their dysregulated expression in the pathogenesis of chronic respiratory diseases, including COPD. Philip M. Hansbro of Centenary Institute and University of Technology Sydney, amd his team performed miRNA microarray analyses of the lungs of mice with CS-induced experimental COPD.[2] miR-21 was the second highest up-regulated miRNA, particularly in airway epithelium and lung macrophages. Its expression in human lung tissue correlated with reduced lung function in COPD. Prophylactic and therapeutic treatment with a specific miR-21 inhibitor (Ant-21) inhibited CS-induced lung miR-21 expression in mice; suppressed airway macrophages, neutrophils, and lymphocytes; and improved lung function, as evidenced by decreased lung hysteresis, transpulmonary resistance(Fig.2). CS exposure reduced lung SATB1 in a mouse model of COPD, whereas Ant-21 treatment restored SATB1 and reduced S100A9 expression and NF-κB activity. Based on these results, specific targeting of lung miR-21 may be a therapeutic approach for COPD.
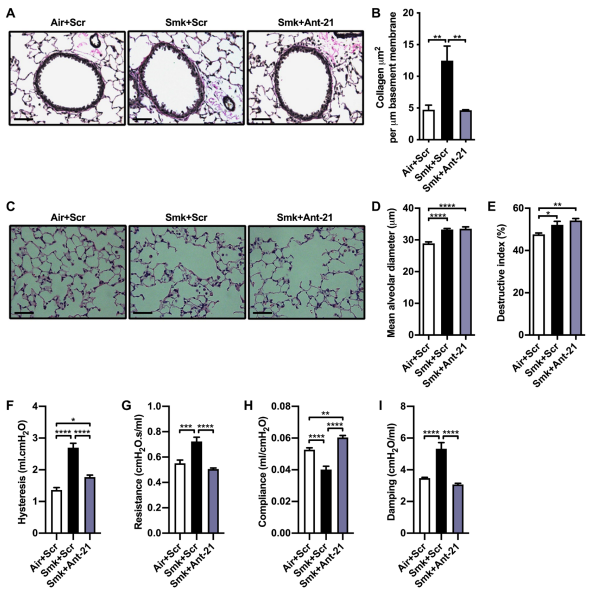
Fig.2 Inhibition of CS-induced lung miR-21 inhibits small-airway remodeling and prevents the impairment of lung function in experimental COPD
3. Inner mitochondrial protein OPA1 plays a role in smoking-related COPD
Mitochondrial dysfunction is the key event which occurs in the pathogenesis of COPD. CS exposure (low vs. high) and duration (acute vs. chronic) are of utmost importance in the mitochondrial damage response. Among the mitochondrial fusion proteins, OPA1 is a sole inner mitochondrial membrane (IMM) protein identified in coordinating fusion events. PA1 has several isoforms, which are mainly divided into two: long (L-) and short (S-) isoforms. Irfan Rahman of University of Rochester Medical Center, and his team were interested in understanding the importance of various OPA1 isoforms and related proteins following CS exposure (acute vs. chronic) and in COPD.[3] Short OPA1 isoforms were predominantly detected and significantly increased in COPD subjects. Acute CS treatment in various cell lines was found to increase the conversion of long to short OPA1 isoforms. CS treatment significantly increased mitochondrial stress-related protein SLP2 in all the cells used. BGP-15 and leflunomide treatment were able to preserve the long OPA1 isoform in cells treated with CS. These results suggest that OPA1 subtype plays a key role in CS-induced lung injury and may serve as a new therapeutic target for COPD(Fig.3).
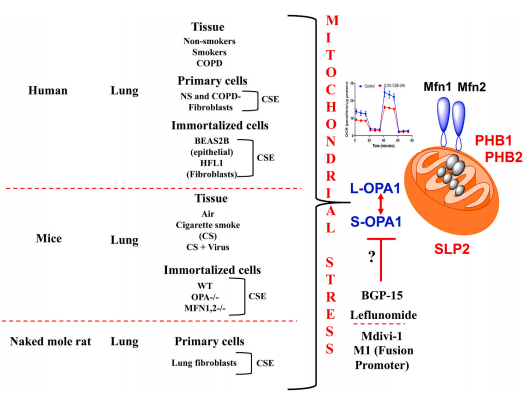
Fig.3 Overall schematic for the involving of OPA1 in mitochondrial dysfunction by CS and in the pathogenesis of COPD
4. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in COPD
Necroptosis, mediated by RIPK3 and MLKL, is a form of regulated necrosis that can drive tissue inflammation and destruction. Zhe Lu of University of Newcastle, and his team tried to determine the role of necroptosis in COPD.[4] The total MLKL protein in the epithelium and macrophages and the pRIPK3 and pMLKL in lung tissue were increased in patients with severe COPD compared with never-smokers or smoker control subjects without COPD. Necroptosis-related mRNA and protein levels were increased in the lungs and macrophages in CS-exposed mice and experimental COPD. Ripk3 or Mlkl deletion prevented airway inflammation upon acute CS exposure. Ripk3 deficiency reduced airway inflammation and remodeling as well as the development of emphysematous pathology after chronic CS exposure(Fig.4). Inhibiting necroptosis attenuates CS-induced airway inflammation, airway remodeling, and emphysema. Targeted inhibition of necroptosis is a potential therapeutic strategy in COPD.
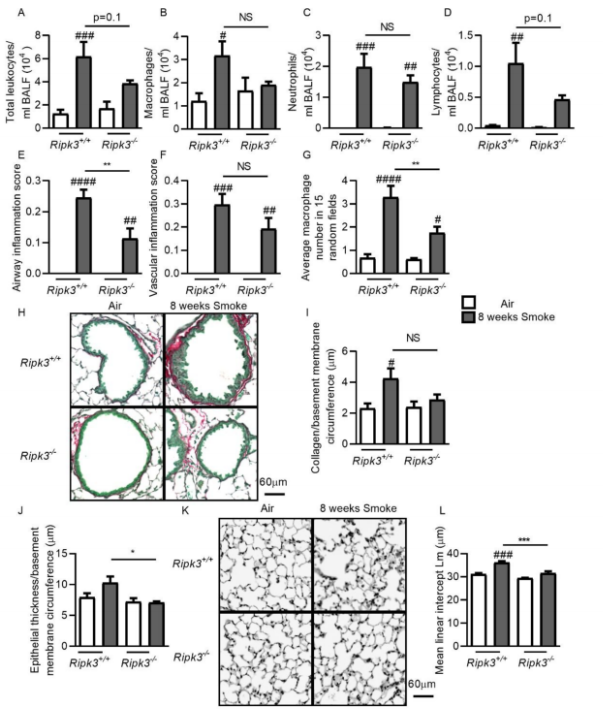
Fig.4 Ripk3 deficiency reduces airway inflammation and remodeling and the development of emphysema pathology after CS exposure
References
[1]Qi H, Liu H, Pullamsetti SS, et al. Epigenetic Regulation by Suv4-20h1 in Cardiopulmonary Progenitor Cells Is Required to Prevent Pulmonary Hypertension and Chronic Obstructive Pulmonary Disease [J]. Circulation. 2021, 144(13):1042-1058.(IF=29.690)
[2]Kim RY, Sunkara KP, Bracke KR, et al. A microRNA-21-mediated SATB1/S100A9/NF-κB axis promotes chronic obstructive pulmonary disease pathogenesis [J]. Sci Transl Med. 2021, 13(621):eaav7223.(IF=17.956)
[3]Maremanda KP, Sundar IK, Rahman I. Role of inner mitochondrial protein OPA1 in mitochondrial dysfunction by tobacco smoking and in the pathogenesis of COPD [J]. Redox Biol. 2021, 45:102055.(IF=11.799)
[4]Lu Z, Van Eeckhoutte HP, Liu G, et al. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in Chronic Obstructive Pulmonary Disease [J]. Am J Respir Crit Care Med. 2021, 204(6):667-681.(IF=21.405)
Cloud-Clone COPD Model performed well in State Key Laboratory
On 10 July, 2020, professor Yiguo Jiang from The First Affiffiffiliated Hospital of Guangzhou Medical University published a paper on Environment International. The paper titled Circular RNA circBbs9 promotes PM2.5-induced lung inflammation in mice via NLRP3 inflammasome activation.
The Chronic obstructive pulmonary disease mice (DSI557Mu01) of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers
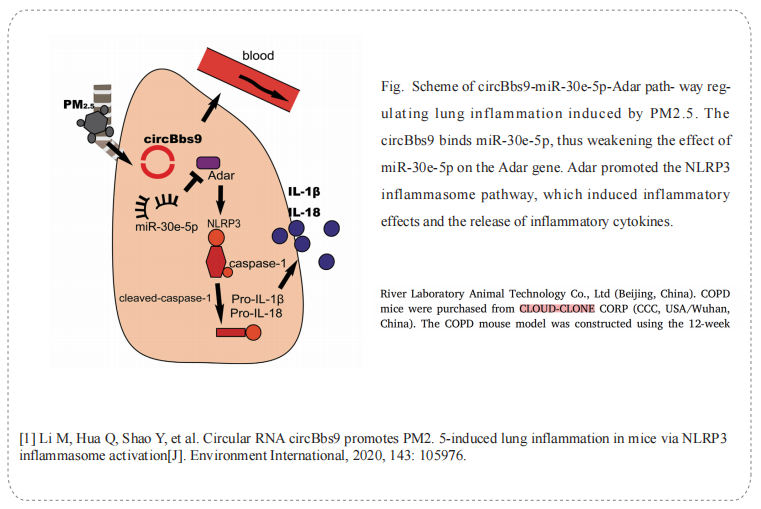
Cloud-Clone not only provides animal models of chronic obstructive pulmonary disease, but also covers animal models of other common respiratory diseases (asthma, bronchitis, pulmonary embolism, pneumonia, pulmonary fibrosis, etc.). It also has various lung disease detection indicators and proteins involved in the above studies (SATB1, S100A9, NF-κB, OPA1, RIPK3, MLKL), which can assist the general scientific research workers in lung disease related research.