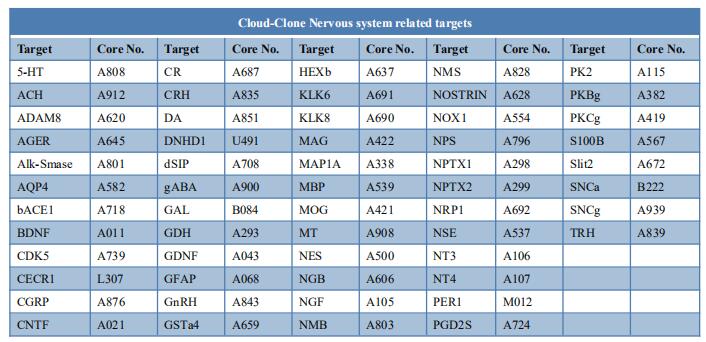
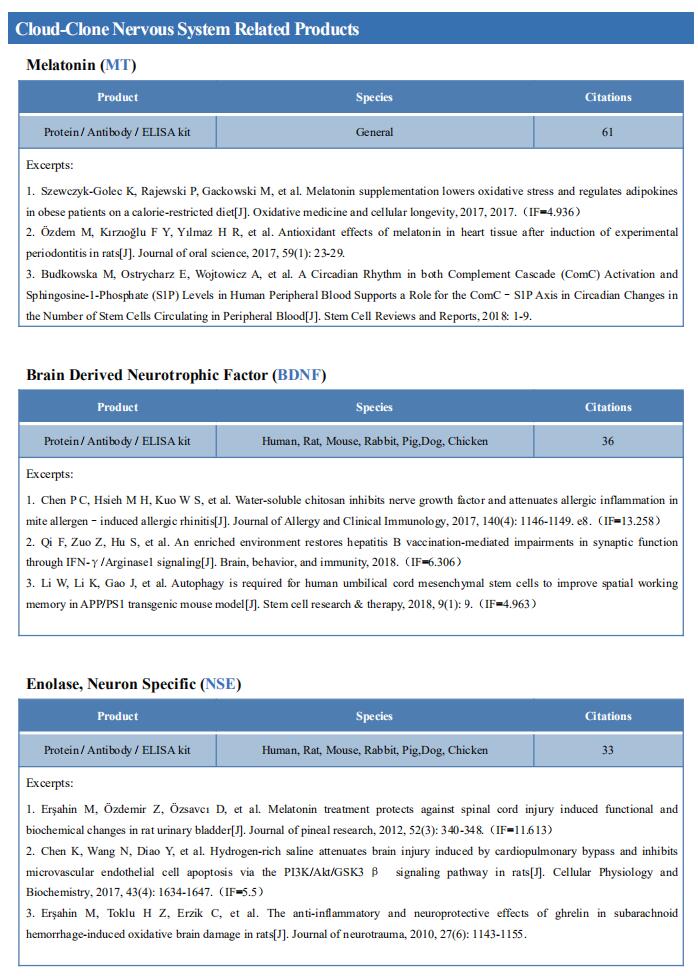
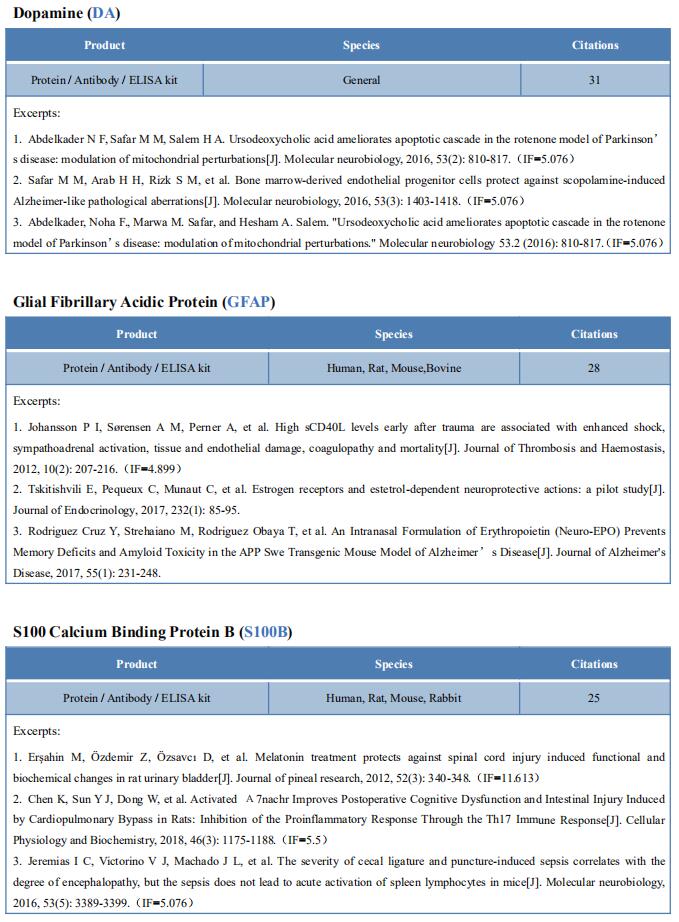
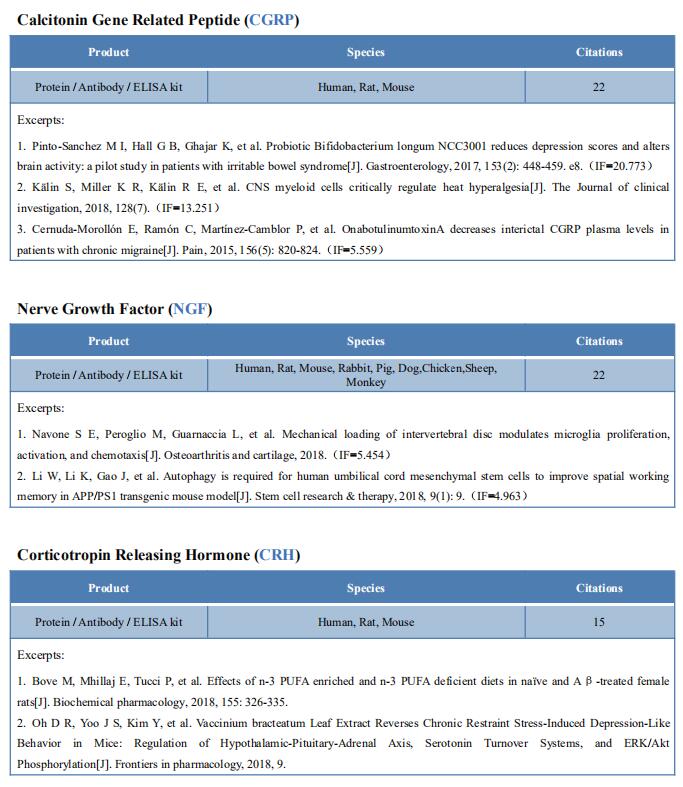
New findings on the mechanism of pain
The International Association for the Study of Pain (IASP) Task Force recently proposed a new definition of pain as an aversive sensory and emotional experience typically caused by, or resembling that caused by, actual or potential tissue injury. Importantly, acute pain serves a critical Darwinian protective function: to initiate an escape response from noxious stimuli that in the future should be avoided for personal safety. However, chronic intractable pain is maladaptive and constitutes a widespread public health issue, significantly impairing quality of life. Pain may arise from multiple mechanisms, and this complexity reflects the difficulty in achieving significant relief. Recently, a number of studies on pain mechanism have been reported, which may provide help for the development of new pain treatment drugs.
1. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain
Both opioids and nonsteroidal anti-inflammatory drugs produce deleterious side effects and fail to provide sustained relief in patients with chronic inflammatory pain. Peripheral neuroinflammation (PN) is critical for initiation and development of inflammatory pain. Ruotian Jiang, Laboratory of Anesthesia and Critical Care Medicine, National-Local Joint Engineering Research Center of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, China, and his team reported the expression of proton-selective ion channel Hv1 in peripheral sensory neurons in rodents and humans[1]. Neuronal Hv1 was up-regulated by PN or depolarizing stimulation, which in turn aggravates inflammation and nociception. Inhibiting neuronal Hv1 genetically or by a newly discovered selective inhibitor YHV98-4 reduced intracellular alkalization and ROS production in inflammatory pain, mitigated the imbalance in downstream SHP-1-pAKT signaling, and also diminished proinflammatory chemokine release to alleviate nociception and morphine-induced hyperalgesia and tolerance (Fig.1). Thus, the data reveal neuronal Hv1 as a novel target in analgesia strategy and managing opioids-related side effects.
2. Microglia-mediated degradation of perineuronal nets promotes pain
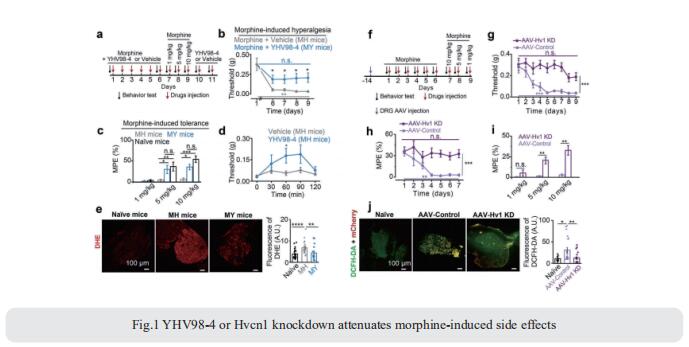
Activation of microglia in the spinal cord dorsal horn after peripheral nerve injury contributes to the development of pain hypersensitivity. Arkady Khoutorsky, Department of Anesthesia, McGill University, Canada, and his team discovered that after peripheral nerve injury, microglia degrade extracellular matrix structures, perineuronal nets (PNNs), in lamina I of the spinal cord dorsal horn (Fig.2)[2]. Lamina I PNNs selectively enwrap spinoparabrachial projection neurons, which integrate nociceptive information in the spinal cord and convey it to supraspinal brain regions to induce pain sensation. Degradation of PNNs by microglia enhances the activity of projection neurons and induces pain-related behaviors. Thus, nerve injury–induced degradation of PNNs is a mechanism by which microglia selectively augment the output of spinal nociceptive circuits and cause pain hypersensitivity.
3. Sodium-calcium exchanger-3 regulates pain ‘‘wind-up’’
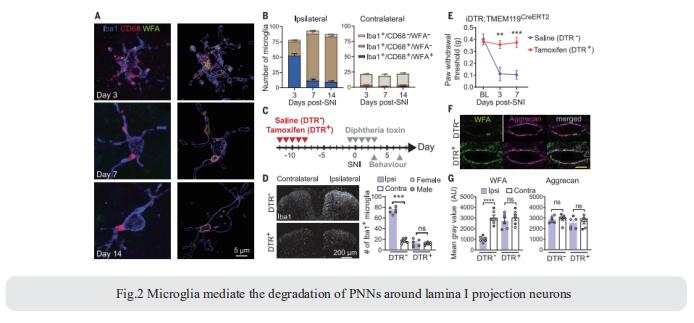
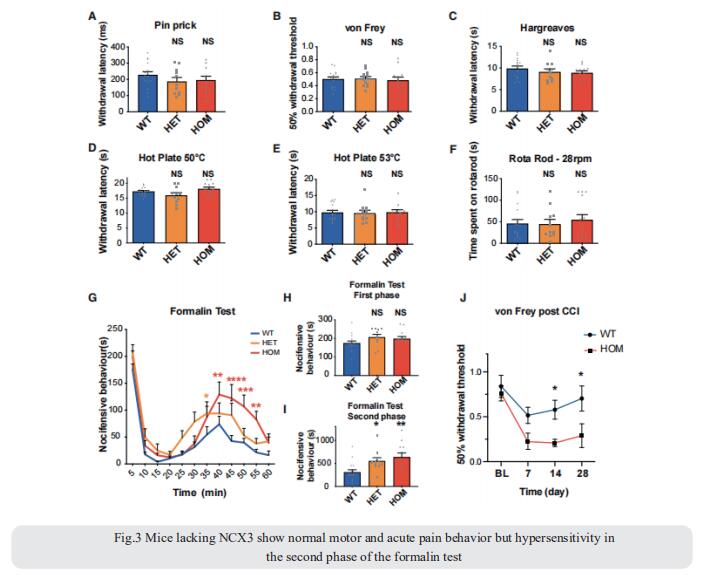
Repeated application of noxious stimuli leads to a progressively increased pain perception; this temporal summation is enhanced in and predictive of clinical pain disorders. Its electrophysiological correlate is ‘‘wind-up,’’ in which dorsal horn spinal neurons increase their response to repeated nociceptor stimulation. To understand the genetic basis of temporal summation, David L. Bennett, Nuffield Department of Clinical Neurosciences, Oxford University, UK, and his team undertook a GWAS of wind-up in healthy human volunteers and found significant association with SLC8A3 encoding sodium-calcium exchanger type 3 (NCX3)[3]. NCX3 was expressed in mouse dorsal horn neurons, and mice lacking NCX3 showed normal, acute pain but hypersensitivity to the second phase of the formalin test and chronic constriction injury (Fig.3). Dorsal horn neurons lacking NCX3 showed increased intracellular calcium following repetitive stimulation, slowed calcium clearance, and increased wind-up. Moreover, virally mediated enhanced spinal expression of NCX3 reduced central sensitization. The study highlights Ca2+ efflux as a pathway underlying temporal summation and persistent pain, which may be amenable to therapeutic targeting.
References
[1]Zhang Q, Ren Y, Mo Y, et al. Inhibiting Hv1 channel in peripheral sensory neurons attenuates chronic inflammatory pain and opioid side effects [J]. Cell Res. 2022;32(5):461-476. (IF=46.297)
[2]Tansley S, Gu N, Guzmán AU, et al. Microglia-mediated degradation of perineuronal nets promotes pain [J]. Science. 2022;377(6601):80-86. (IF=63.714)
[3]Trendafilova T, Adhikari K, Schmid AB, et al. Sodium-calcium exchanger-3 regulates pain "wind-up": From human psychophysics to spinal mechanisms [J]. Neuron. 2022;110(16):2571-2587.e13. (IF=18.688)
Cloud-Clone can not only provide animal models of pain diseases such as migraine, tooth trigger pain and trigeminal neuralgia,, but also have a variety of inflammatory and pain stress related serum indicators detection products and the above SHP-1-pAKT signaling pathway related products, which can help researchers to carry out pain mechanism related research.