New progress in Parkinson's disease research
Parkinson’s disease (PD) is the second most common neurodegenerative disease, affecting millions of individuals. These motor signs are usually preceded by non-motor manifestations such as olfactory dysfunction, rapid eye movement sleep behaviour disorder, depression, and constipation. Additionally, over the course of the disease, cognitive impairment also develops with progression to dementia in up to 80% of the patients.
New progress in Parkinson's disease research
The cardinal motor symptoms of PD are caused by the loss of dopaminergic neurons in the substantia nigra that modulate basal ganglia circuits regulating habitual and goal-directed movement. The pathogenesis of PD is undoubtedly multifactorial. Various cellular dysfunctions including altered dopamine metabolism, increased oxidative stress, mitochondrial failure, altered calcium homeostasis, neuroinflammation, impaired autophagy and proteasome dysfunctions appear tightly linked to PD-associated neuronal loss. Furthermore, numerous evidence indicate that the intracellular accumulation of misfolded proteins notably SNCA/alpha-synuclein and endoplasmic reticulum overload could contribute to this pathology.
1. Disruption of mitochondrial complex I induces progressive PD
Loss of functional mitochondrial complex I (MCI) in the dopaminergic neurons of the substantia nigra is a hallmark of Parkinson’s disease. D. James Surmeier, Feinberg School of Medicine, Northwestern University, and his team deleted the gene encoding an essential subunit of the MCI catalytic core, Ndufs2, in dopaminergic neurons using intersectional genomics[1]. Disruption of MCI induced a Warburg-like shift in metabolism that enabled neuronal survival, but triggered a progressive loss of the dopaminergic phenotype that was frst evident in nigrostriatal axons. This axonal defcit was accompanied by motor learning and fne motor defcits, but not by clear levodopa-responsive parkinsonism—which emerged only after the later loss of dopamine release in the substantia nigra (Fig.1). Thus, MCI dysfunction alone is sufcient to cause progressive, human-like parkinsonism in which the loss of nigral dopamine release makes a critical contribution to motor dysfunction, contrary to the current Parkinson’s disease paradigm.
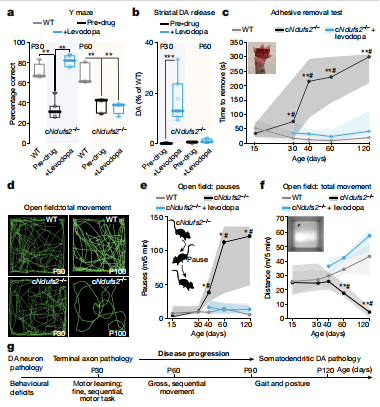
Fig.1 Ndufs2−/− mice present progressive, levodopa-responsive PD
2. LRP10 interacts with SORL1 in the intracellular vesicle trafcking pathway, which plays a role in PD progression
Loss-of-function variants in the low-density lipoprotein receptor-related protein 10 (LRP10) gene have been associated with autosomal-dominant PD and PD dementia.To better understand how LRP10 variants lead to neurodegeneration, Wim Mandemakers, University Medical Center Rotterdam, and his team first performed an in-depth characterisation of LRP10 expression in post-mortem brains and human-induced pluripotent stem cell (iPSC)-derived astrocytes and neurons from control subjects[2]. In adult human brain and iPSC-derived cells, LRP10 is mainly expressed in astrocytes but undetectable in neurons. In astrocytes, LRP10 is present at trans-Golgi network, plasma membrane, retromer, and early endosomes. Interestingly, LRP10 also partially co-localises and interacts with sortilin-related receptor 1 (SORL1) (Fig.2). Furthermore, signifcantly enlarged LRP10-positive vesicles were detected in a patient carrying the LRP10 variant. It was further found that LRP10 positive vesicles were the core of mature lewy bodies in the substantia nigra of PD brain. High LRP10 expression in non-neuronal cells and undetectable levels in neurons of control subjects indicate that LRP10-mediated pathogenicity is initiated via cell non-autonomous mechanisms, potentially involving the interaction of LRP10 with SORL1 in vesicle trafcking pathways.
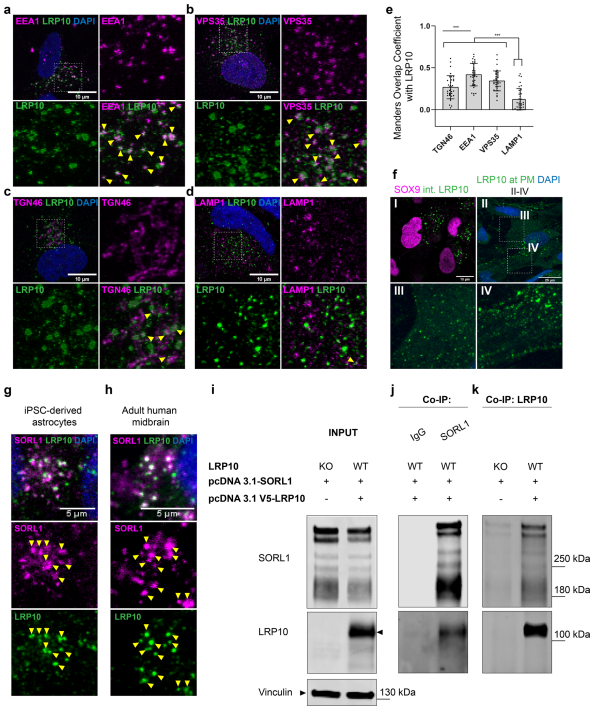
Fig.2 LRP10 is localised to vesicular structures and interacts with SORL1
3. The functional interplay between XBP1s and PINK1 governs mitophagy and potentially impacts PD pathophysiology
PD-affected brains show consistent endoplasmic reticulum (ER) stress and mitophagic dysfunctions. XBP1 is a transcription factor activated upon ER stress after unconventional splicing by the nuclease ERN1/IREα thereby yielding XBP1s, whereas PINK1 is a kinase considered as the sensor of mitochondrial physiology and a master gatekeeper of mitophagy process. Cristine Alves da Costa, Université Côte d’Azur, Valbonne, France, and his team showed that XBP1s transactivates PINK1 in human cells, primary cultured neurons and mice brain, and triggered a pro-mitophagic phenotype that was fully dependent of endogenous PINK1[3]. They also unraveled a PINK1-dependent phosphorylation of XBP1s that conditioned its nuclear localization and thereby, governed its transcriptional activity(Fig.3). PINK1-induced XBP1s phosphorylation occurred at residues reminiscent of, and correlated to, those phosphorylated in substantia nigra of sporadic PD-affected brains. The study delineated a functional loop between XBP1s and PINK1 governing mitophagy that was disrupted in PD condition.
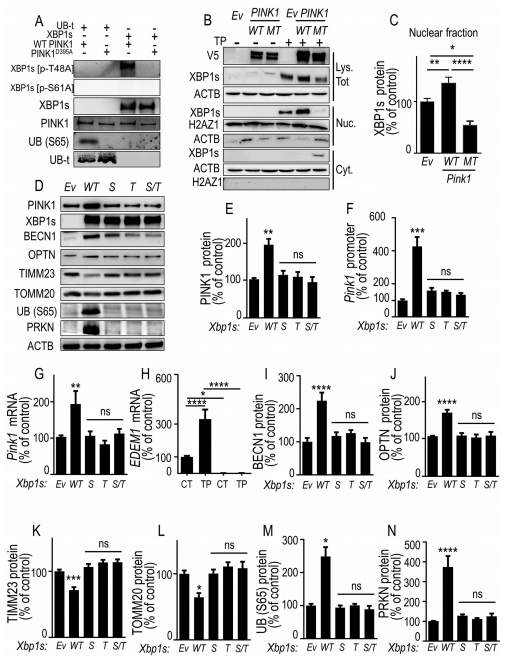
Fig.3 PINK1-mediated phosphorylation of XBP1s enhances its nuclear translocation, controls its own transcription and mitophagy
References
[1] González-Rodríguez P, Zampese E, Stout KA, et al. Disruption of mitochondrial complex I induces progressive parkinsonism [J]. Nature. 2021;599(7886):650-656. (IF=49.962)
[2] Grochowska MM, Carreras Mascaro A, Boumeester V, et al. LRP10 interacts with SORL1 in the intracellular vesicle trafficking pathway in non-neuronal brain cells and localises to Lewy bodies in Parkinson's disease and dementia with Lewy bodies [J]. Acta Neuropathol. 2021;142(1):117-137.(IF=17.088)
[3] El Manaa W, Duplan E, Goiran T, et al. Transcription- and phosphorylation-dependent control of a functional interplay between XBP1s and PINK1 governs mitophagy and potentially impacts Parkinson disease pathophysiology. Autophagy. 2021;17(12):4363-4385.(IF=16.016)
Cloud-Clone can not only provide animal models of various neurological diseases, including Parkinson's disease, Alzheimer's disease, anxiety disorder, chronic stress depression, etc., cover common neurological diseases. It also has various neurological disease detection indicators related products, which can help the majority of scientific researchers to carry out neurological disease related research.