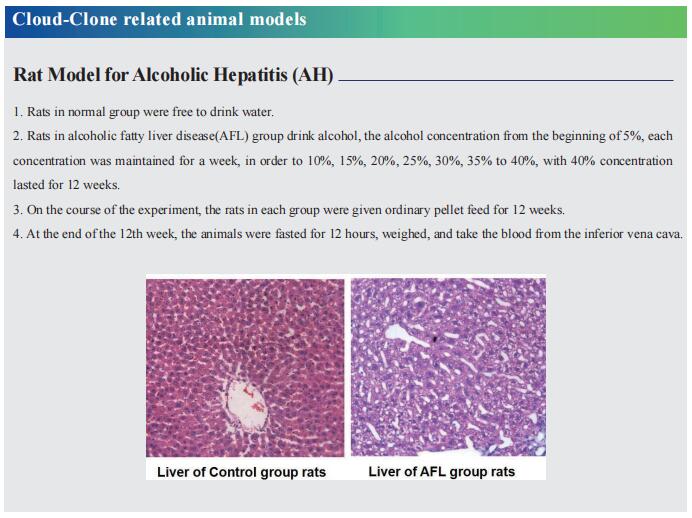
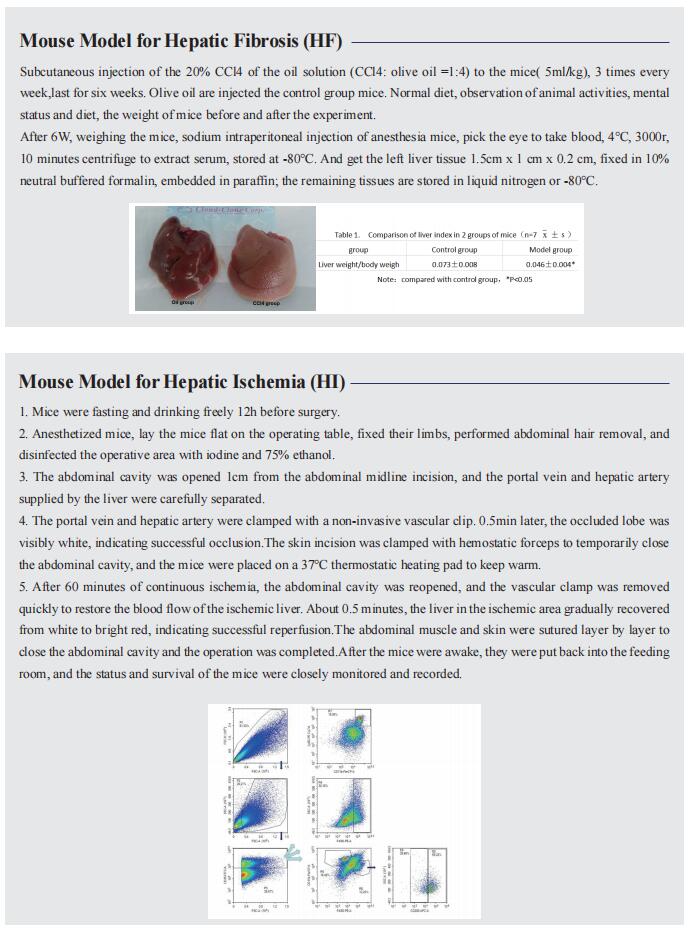
Study of bioengineered liver tissue for treatment of liver diseases
The liver is an essential organ for maintaining normal life activities of the human body, because it not only regulates the metabolism of many nutrients and chemical drugs, but also has many functions such as synthesizing and decomposing proteins, regulating systemic blood volume, excluding body toxins, and regulating immunity. Based on the different etiologies and pathogenesis, liver diseases are classified as acute liver injury, viral hepatitis, alcoholic liver disease, metabolic-associated fatty liver disease, liver fibrosis, cirrhosis, and hepatocellular carcinoma. Due to the changes of living environment and the irregularity of life habits, the number of patients with liver diseases is increasing worldwide in recent years, which has gradually developed into a global public health problem. Recently, multiple studies have reported on bioengineered liver tissue, which may be of great significance in elucidating the pathogenesis of liver disease, developing targeted therapeutic drugs, and improving clinical treatment of liver disease.
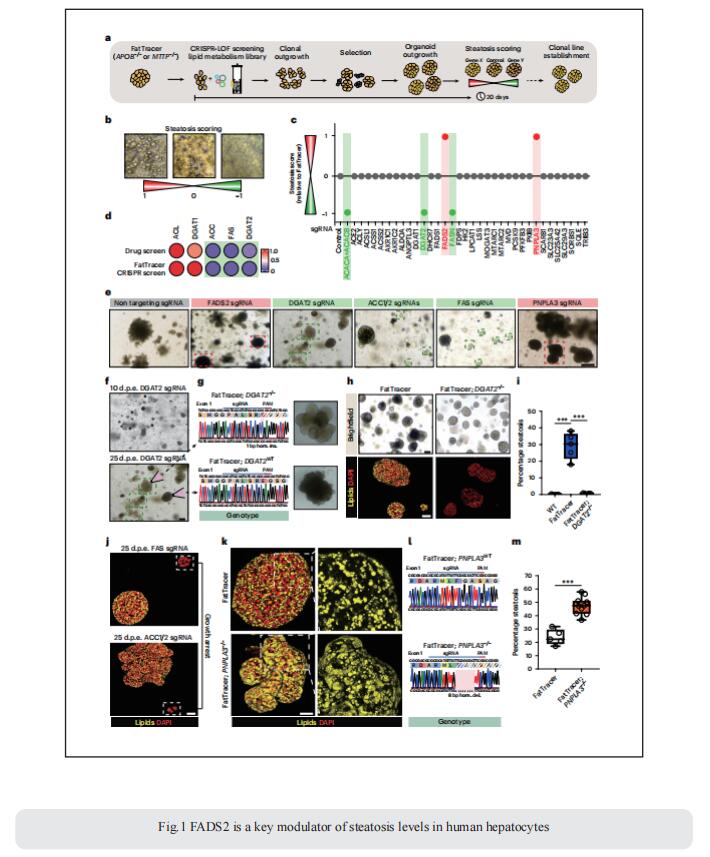
1. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis
The lack of registered drugs for nonalcoholic fatty liver disease (NAFLD) is partly due to the paucity of human-relevant models for target discovery and compound screening. Delilah Hendriks, Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences, The Netherlands, and his team used human fetal hepatocyte organoids to model the frst stage of NAFLD, steatosis, representing three diferent triggers: free fatty acid loading, interindividual genetic variability (PNPLA3 I148M) and monogenic lipid disorders (APOB and MTTP mutations)[1]. They performed drug screening and identified compounds effective at resolving steatosis across these different models, while highlighting that PNPLA3 I148M impairs drug response. They leveraged the APOB- and MTTP-mutant organoids to establish a CRISPR-based screening platform to identify steatosis modulators/targets and to evaluate NAFLD risk genes. This platform, termed FatTracer, identified FADS2 as a critical steatosis modulator (Fig.1). Enhancement of FADS2 expression increases polyunsaturated fatty acid abundancy which, in turn, reduces de novo lipogenesis. These organoid models facilitate study of steatosis etiology and drug targets.
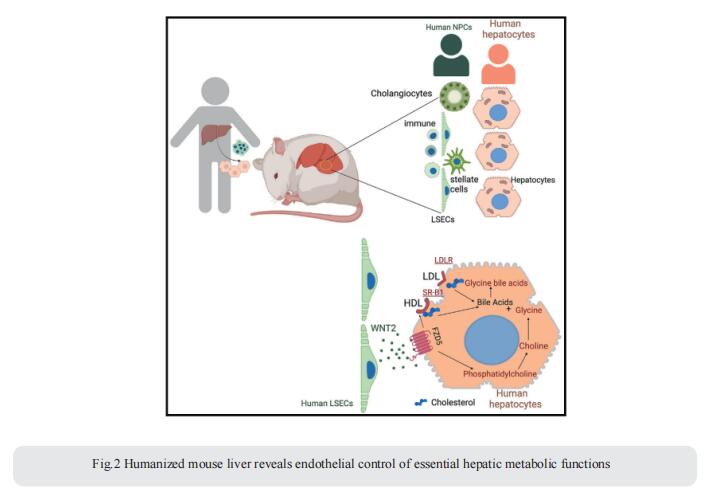
2. Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions
In chronic liver diseases, hepatocyte malfunction represents a major cause of morbidity and mortality. Thus, understanding how hepatocyte function is regulated in the dynamic environment of the liver is crucial to understanding the mechanisms underlying hepatocyte dysfunction in the setting of chronic liver disease. Richard A. Flavell, Department of Immunobiology, Yale School of Medicine, USA, and his team showed that key metabolic functions of human hepatocytes are controlled by non-parenchymal cells (NPCs) in their microenvironment[2]. They developed mice bearing human hepatic tissue composed of human hepatocytes and NPCs, including human immune, endothelial, and stellate cells. Humanized livers reproduce human liver architecture, perform vital human-specific metabolic/homeostatic processes, and model human pathologies, including fibrosis and NAFLD. Leveraging species mismatch and lipidomics, they demonstrate that human NPCs control metabolic functions of human hepatocytes in a paracrine manner. Mechanistically, they uncover a species-specific interaction whereby WNT2 secreted by sinusoidal endothelial cells controls cholesterol uptake and bile acid conjugation in hepatocytes through receptor FZD5 (Fig.2). This study establishes an important technological platform and an experimental approach to study cell-to-cell interactions in the human liver in vivo, in homeostasis, and in diseases such as NAFLD and fibrosis and a new tool to identify and evaluate therapeutic targets.
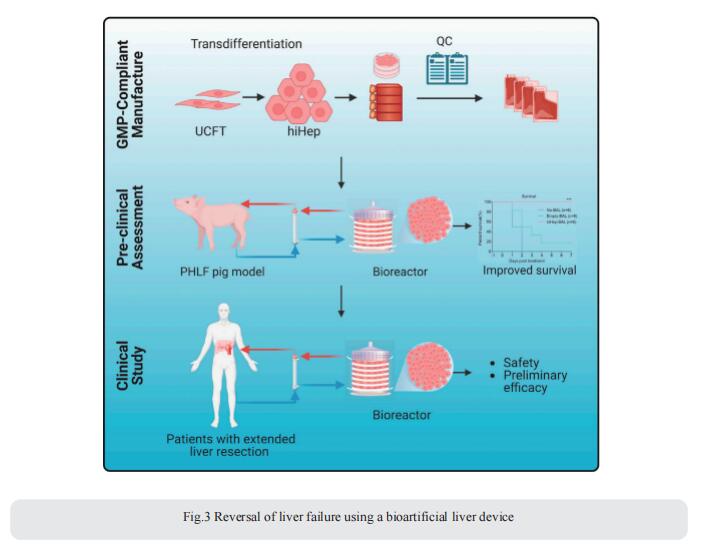
3. Reversal of liver failure using a bioartificial liver device
Liver resection is the first-line treatment for primary liver cancers, providing the potential for a cure. However, concerns about post-hepatectomy liver failure (PHLF), a leading cause of death following extended liver resection, have restricted the population of eligible patients. Xiujun Cai, Department of General Surgery, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, China, and his team engineered a clinical-grade bioartificial liver (BAL) device employing human-induced hepatocytes (hiHeps) manufactured under GMP conditions[3]. In a porcine PHLF model, the hiHep-BAL treatment showed a remarkable survival benefit. On top of the supportive function, hiHep-BAL treatment restored functions, specifically ammonia detoxification, of the remnant liver and facilitated liver regeneration (Fig.3). Notably, an investigator-initiated study in seven patients with extended liver resection demonstrated that hiHep-BAL treatment was well tolerated and associated with improved liver function and liver regeneration, meeting the primary outcome of safety and feasibility. These encouraging results warrant further testing of hiHep-BAL for PHLF, the success of which would broaden the population of patients eligible for liver resection.
References
[1]Hendriks D, Brouwers JF, Hamer K, et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis [J]. Nat Biotechnol. 2023;10.1038/s41587-023-01680-4. (IF=46.9)
[2]Kaffe E, Roulis M, Zhao J, et al. Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions [J]. Cell. 2023;S0092-8674(23)00795-X. (IF=64.5)
[3]Wang Y, Zheng Q, Sun Z, et al. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes [J]. Cell Stem Cell. 2023;30(5):617-631.e8. (IF=23.9)
Cloud-Clone not only provides animal models of various liver diseases, including liver fibrosis, liver ischemia, alcoholic liver disease, intrahepatic cholestasis, liver cirrhosis, fatty liver, liver failure, etc., covering common liver diseases. We also have a variety of commonly used detection indicators for liver diseases and the above-mentioned protein-related products such as APOB, MTTP, FADS2, WNT2, FZD, etc., which can help scientific researchers to carry out liver disease research.