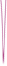Targeted regulatory T cell immunotherapy for hepatocellular carcinoma
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) accounts for approximately 90% of primary liver cancers and ranks as the fourth most prevalent cancer worldwide. HCC primarily develops from hepatitis C virus infection, alcohol use, or nonalcoholic steatohepatitis (NASH) in North America and Europe; however, in eastern Asia and Africa, hepatitis B virus (HBV) infection is the predominant etiological factor.
Regulatory T cells
Regulatory T cells (Treg) are a subset of T cells that control autoimmune reactivity in vivo. It has been a research hotspot in the field of immunology in recent years. Treg has the two major characteristics of low immune response and immunosuppression. Through an "active" approach suppress the immune system,playing the role of negative immune regulation.
Treg and NASH-associated HCC
Increased infiltration of Tregs into the tumor tissue of patients with HCC has long been observed and suggested as both a potential prognostic marker and therapeutic intervention target. However, a clear mechanistic understanding of the roles of Tregs in the premalignant process from NASH to HCC development is still lacking. On 4 August, 2021, an article published by Allan Tsung of the Ohio State University Wisner Medical Center, Dean Tian from Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology and their team in Journal of Hepatology reveals this mechanism(Fig 1)[1]. In the study, they found that Tregs and neutrophil extracellular traps(NETs) play a pivotal role in carcinogenesis in the context of NASH-HCC, and increased intrahepatic Tregs contribute to HCC initiation and progression in NASH livers. Tregs are regulated by NETs, which promote mitochondrial OXPHOS and facilitates Treg differentiation from naïve CD4+ T cells. In addition, NET-related metabolic reprogramming in naïve CD4+ T cells requires toll-like receptor 4 (TLR4). These data also uncover crosstalk between adaptive and innate immunity through NETs, which exposes a potential therapeutic intervention target for NASH-HCC. Several new questions arise from these findings, which require further investigation. For example, more studies are needed to understand what components of the NETs structure interact with TLR4 on CD4+ T cells and, importantly, the nature of the downstream pathways. More work is also needed to clarify how NETs affect Treg suppressive function in vivo.
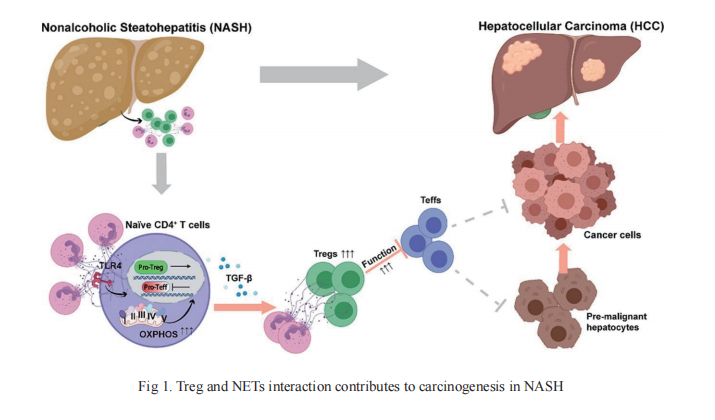
Treg and HBV-related HCC
HBV infection is an established major risk factor for HCC development, often following from, or accompanying long-term chronic inflammation. The incidence of HCC increases with increasing viral load and duration of infection, suggesting an accumulating risk of tumorigenesis from this chronic inflammatory state. On 12 September, 2021, Pengyuan Yang from the Institute of Biophysics, University of Chinese Academy of Sciences, Fu-Sheng Wang from the Fifth Medical Center of the Chinese People's Liberation Army General Hospital and their team published an article in Journal of Hepatology that clarified the immunosuppressive effect of Treg in HBV+ HCC(Fig 2)[2]. The CC chemokine receptor 4 (CCR4), expressed on Tregs and other T helper cells (Th), binds to its ligands CCL22 and CCL17, and is a key chemokine receptor mediating Treg trafficking into the tumor microenvironment(TME). CCR4+ Tregs were more immunosuppressive than CCR4- Tregs, and were characterized as having PD-1+TCF1+ stem-like properties, and mediating the cell resource of immunosuppression and immune escape in HBV+ HCC. Furthermore, CCR4+ Tregs were more efficient than CCR4- Tregs at suppressing T cell function in vitro and in vivo, potentially through upregulating TCF1, PD-1, and CTLA-4 levels, and secreting more immunosuppressive cytokines, such as IL-10 and IL-35. Antagonism of CCR4 overcame sorafenib resistance and strongly sensitized tumors to checkpoint blockade by specifically targeting tumor-infiltrating Tregs to initiate T-cell reactivation. And the article published on "SCIENCE TRANSLATIONAL MEDICINE" on 4 August , 2021 shows that similar expression patterns of CTLA-4 and CD47 on Treg cells in patients with cancer[3]. Dual targeting of CTLA-4 and CD47 on tumor Treg cells with the anti–CTLA-4×SIRPaheterodimer is a potent strategy to selectively deplete intratumoral Tregcells. This is accomplished by simultaneously enhancing an eat me signal and blocking a do not eat me signal. It may be an effective strategy for treating solid tumors. Which suggests that targeting CCR4 could be a new immunotherapeutic strategy with wide application prospects that complements both first and second-line agents against HCC.
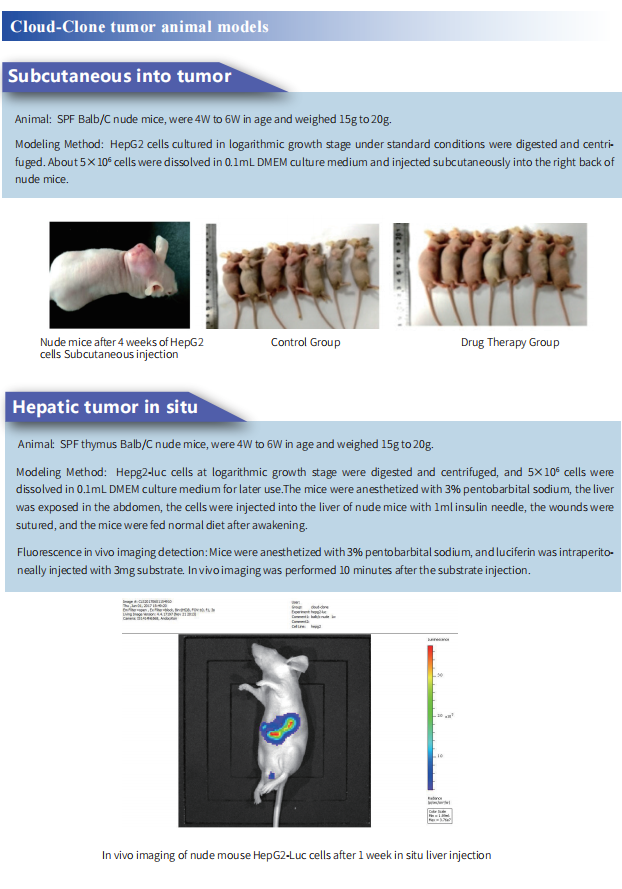
Cloud-Clone can provide a variety of tumor experimental animal models, including tumor transplantation animal models, spontaneous tumor animal models, induced tumor animal models, tumor metastasis animal models, etc., covering common tumor research. And we also has various cancer detection indicators and the above-mentioned Treg-mediated immunosuppressive pathways (TLR4, CCR4, CCL22, CCL17, CTLA-4, PD-1) related products, which can help the majority of scientific researchers to carry out cancer immunotherapy related research.
Reference
[1] Wang H, Zhang H, Wang Y, et al. Regulatory T cell and neutrophil extracellular trap interaction contributes to carcinogenesis in non-alcoholic steatohepatitis[J]. Journal of Hepatology, 2021, Aug 4:S0168-8278(21)01962-0.(IF=25.083)
[2] Gao Y, You M, Fu J, et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B[J]. Journal of Hepatology, 2021,DOI:https://doi.org/10.1016/j.jhep.2021.08.029.(IF=25.083)
[3] Zhang A, Ren Z, Tseng KF, et al. Dual targeting of CTLA-4 and CD47 on T cells promotes immunity against solid tumors[J] .Science Translational Medicine, 2021, 13: eabg8693.(IF=17.956)