Rat Model for Preeclampsia (PE) 
Pre-eclampsia
- UOM
- FOB US$ 260.00
- Quantity
Overview
Properties
- Product No.DSI779Ra01
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- ApplicationsDisease Model
Research use only - Downloadn/a
- CategoryCerebral and nervous systems
- Prototype SpeciesHuman
- SourceInduced by injection of L-NAME
- Model Animal StrainsSD rats (SPF), female, pregnant for 18 days
- Modeling GroupingRandomly divided into six group: Control group, Model group, Positive drug group and Test drug group
- Modeling Period3 weeks
Share your citation
Upload your experimental result
Review
Leave a message
Loading...
Sign into your account
Share a new citation as an author
Upload your experimental result
Review
Please attach serial No. on instruction manual


Contact us
Please fill in the blank.
Name*
Organization
Address
E-mail address*
Telephone
Inquiry*
Verification code*
Modeling Method
The 18 day of pregnancy in female rats were subcutaneously injected with L-nitro-arginine (L-NAME) for 4 days.
Model evaluation
Pathological results
Cytokines level
Statistical analysis
SPSS software is used for statistical analysis, measurement data to mean ± standard deviation (x ±s), using t test and single factor analysis of variance for group comparison , P<0.05 indicates there was a significant difference, P<0.01 indicates there are very significant differences.
Giveaways
Increment services
-
 Tissue/Sections Customized Service
Tissue/Sections Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 Immunohistochemistry (IHC) Experiment Service
Immunohistochemistry (IHC) Experiment Service
-
 Small Animal In Vivo Imaging Experiment Service
Small Animal In Vivo Imaging Experiment Service
-
 Small Animal Micro CT Imaging Experiment Service
Small Animal Micro CT Imaging Experiment Service
-
 Small Animal MRI Imaging Experiment Service
Small Animal MRI Imaging Experiment Service
-
 Small Animal Ultrasound Imaging Experiment Service
Small Animal Ultrasound Imaging Experiment Service
-
 Transmission Electron Microscopy (TEM) Experiment Service
Transmission Electron Microscopy (TEM) Experiment Service
-
 Scanning Electron Microscope (SEM) Experiment Service
Scanning Electron Microscope (SEM) Experiment Service
-
 Learning and Memory Behavioral Experiment Service
Learning and Memory Behavioral Experiment Service
-
 Anxiety and Depression Behavioral Experiment Service
Anxiety and Depression Behavioral Experiment Service
-
 Drug Addiction Behavioral Experiment Service
Drug Addiction Behavioral Experiment Service
-
 Pain Behavioral Experiment Service
Pain Behavioral Experiment Service
-
 Neuropsychiatric Disorder Behavioral Experiment Service
Neuropsychiatric Disorder Behavioral Experiment Service
-
 Fatigue Behavioral Experiment Service
Fatigue Behavioral Experiment Service
-
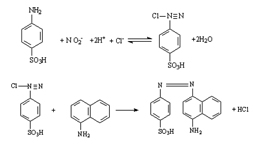 Nitric Oxide Assay Kit (A012)
Nitric Oxide Assay Kit (A012)
-
 Nitric Oxide Assay Kit (A013-2)
Nitric Oxide Assay Kit (A013-2)
-
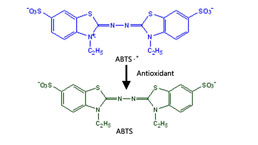 Total Anti-Oxidative Capability Assay Kit(A015-2)
Total Anti-Oxidative Capability Assay Kit(A015-2)
-
 Total Anti-Oxidative Capability Assay Kit (A015-1)
Total Anti-Oxidative Capability Assay Kit (A015-1)
-
 Superoxide Dismutase Assay Kit
Superoxide Dismutase Assay Kit
-
 Fructose Assay Kit (A085)
Fructose Assay Kit (A085)
-
 Citric Acid Assay Kit (A128 )
Citric Acid Assay Kit (A128 )
-
 Catalase Assay Kit
Catalase Assay Kit
-
 Malondialdehyde Assay Kit
Malondialdehyde Assay Kit
-
 Glutathione S-Transferase Assay Kit
Glutathione S-Transferase Assay Kit
-
 Microscale Reduced Glutathione assay kit
Microscale Reduced Glutathione assay kit
-
 Glutathione Reductase Activity Coefficient Assay Kit
Glutathione Reductase Activity Coefficient Assay Kit
-
 Angiotensin Converting Enzyme Kit
Angiotensin Converting Enzyme Kit
-
 Glutathione Peroxidase (GSH-PX) Assay Kit
Glutathione Peroxidase (GSH-PX) Assay Kit
-
 Cloud-Clone Multiplex assay kits
Cloud-Clone Multiplex assay kits